Home /
Expert Answers /
Physics /
the-dipole-moment-of-the-water-molecule-h2o-is-6-171030cm-consider-a-water-molecule-l-pa306
(Solved): The dipole moment of the water molecule (H2O) is 6.171030Cm. Consider a water molecule l ...
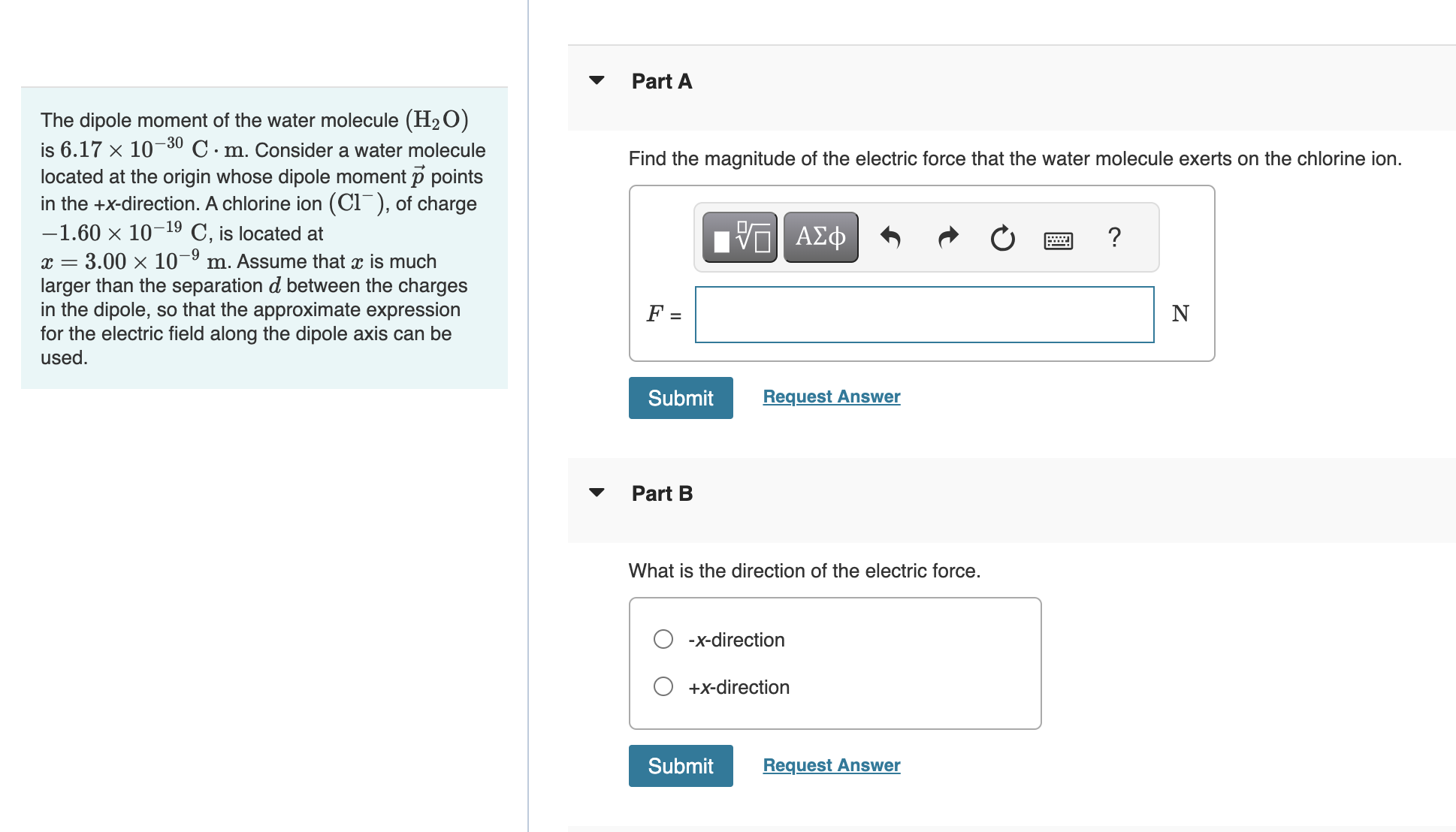
The dipole moment of the water molecule is . Consider a water molecule located at the origin whose dipole moment points in the -direction. A chlorine ion , of charge , is located at . Assume that is much larger than the separation between the charges in the dipole, so that the approximate expression for the electric field along the dipole axis can be used. Find the magnitude of the electric force that the water molecule exerts on the chlorine ion. Part B What is the direction of the electric force.
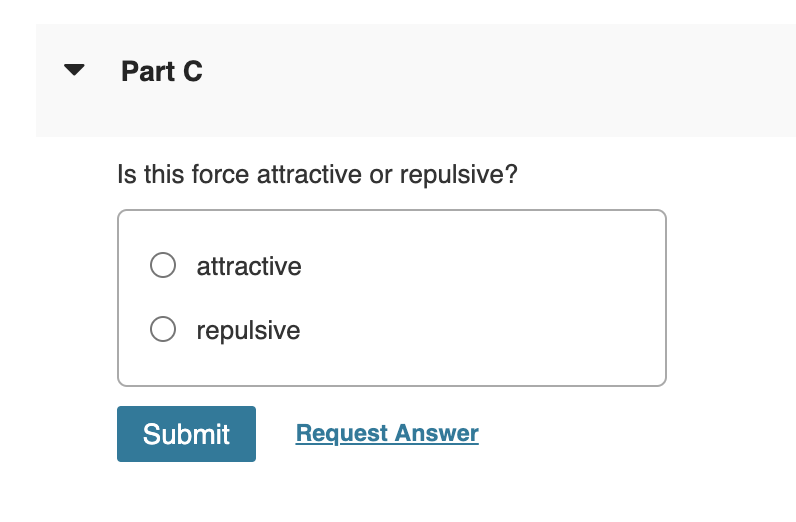
Is this force attractive or repulsive? attractive repulsive