Home /
Expert Answers /
Chemistry /
the-difference-between-the-mass-number-a-of-an-atom-and-the-atomic-number-mathrm-z-pa796
(Solved): The difference between the mass number, \( A \), of an atom and the atomic number, \( \mathrm{Z} \) ...
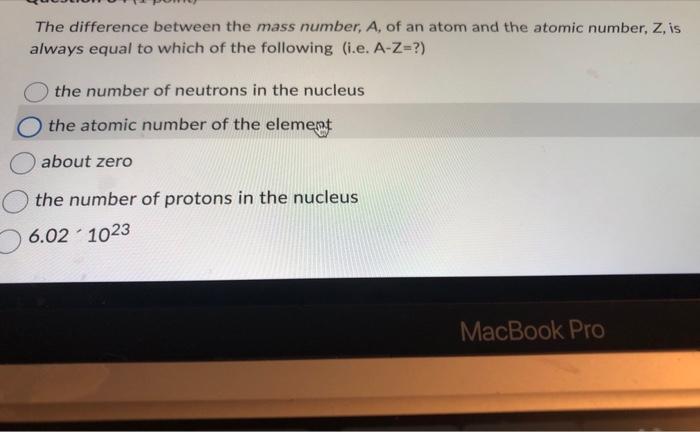
The difference between the mass number, \( A \), of an atom and the atomic number, \( \mathrm{Z} \), is always equal to which of the following (i.e. \( A-Z= \) ?) the number of neutrons in the nucleus the atomic number of the element about zero the number of protons in the nucleus \[ 6.02-10^{23} \]