Home /
Expert Answers /
Chemistry /
the-diagram-below-represents-vessels-i-ii-iii-of-the-same-volume-and-held-at-the-same-temperature-pa305
(Solved): The diagram below represents vessels I, II, III of the same volume and held at the same temperature ...
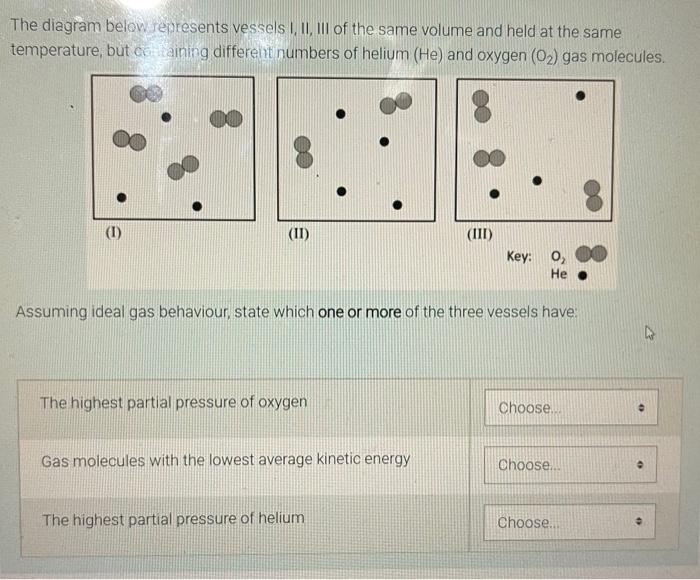
The diagram below represents vessels I, II, III of the same volume and held at the same temperature, but containing different numbers of helium (He) and oxygen (0?) gas molecules. (1) (II) (III) Key: 0? He Assuming ideal gas behaviour, state which one or more of the three vessels have: The highest partial pressure of oxygen Choose... Gas molecules with the lowest average kinetic energy Choose.... The highest partial pressure of helium Choose.. 0
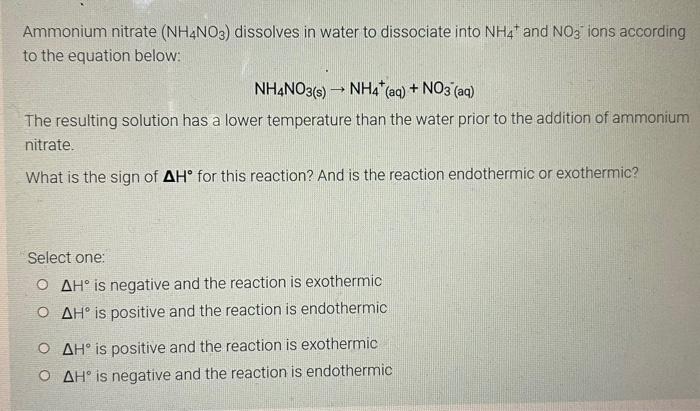
Ammonium nitrate (NH4NO3) dissolves in water to dissociate into NH4 and NO3 ions according to the equation below: NH4NO3(s) ? NH4* (aq) + NO3(aq) The resulting solution has a lower temperature than the water prior to the addition of ammonium nitrate. What is the sign of AH° for this reaction? And is the reaction endothermic or exothermic? Select one: ? ??° is negative and the reaction is exothermic O AH is positive and the reaction is endothermic ? ??° is positive and the reaction is exothermic ? ??° is negative and the reaction is endothermic
Expert Answer
Answer:- This question is answere