Home /
Expert Answers /
Chemistry /
the-decomposition-of-carbon-dioxide-proceeds-as-follows-2-mathrm-co-2-mathrm-g-text-pa276
(Solved): The decomposition of carbon-dioxide proceeds as follows: \[ 2 \mathrm{CO}_{2}(\mathrm{~g})+\text { ...
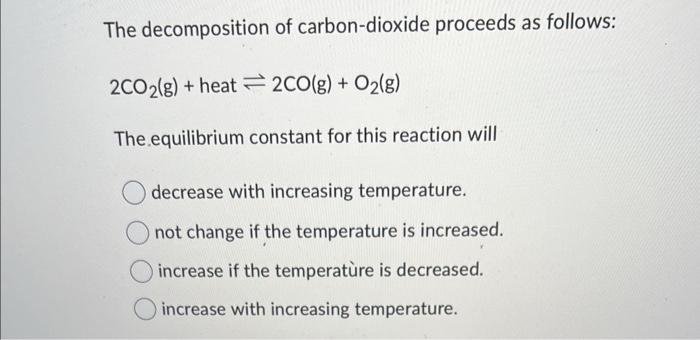
The decomposition of carbon-dioxide proceeds as follows: \[ 2 \mathrm{CO}_{2}(\mathrm{~g})+\text { heat } \rightleftharpoons 2 \mathrm{CO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \] The equilibrium constant for this reaction will decrease with increasing temperature. not change if the temperature is increased. increase if the temperatùre is decreased. increase with increasing temperature.
Expert Answer
* As heat is absorbed, it is an endothermic reaction. * From Le Chatelier's Principle, if a system under equilibrium be subjected to a change in temperature, pressure or concentrat