Home /
Expert Answers /
Chemical Engineering /
the-copper-in-an-aqueous-sample-was-determined-by-aas-first-19-0-ml-of-unknown-were-pipetted-into-pa393
(Solved): The copper in an aqueous sample was determined by AAS. First, 19.0 mL of unknown were pipetted into ...
The copper in an aqueous sample was determined by AAS.
First, 19.0 mL of unknown were pipetted into each of five 50.0 mL volumetric flask.
Various volumes of standard containing 12.2 ppm Cu were added to the flasks and the solution were then diluted to volume.
1. Calculate the concentration of standard Cu in each flask
2. Plot the graph of absorbance vs concentration of standard
3. Determine the linear equation of the data
4. Find the concentration of the sample based on the equation obtain
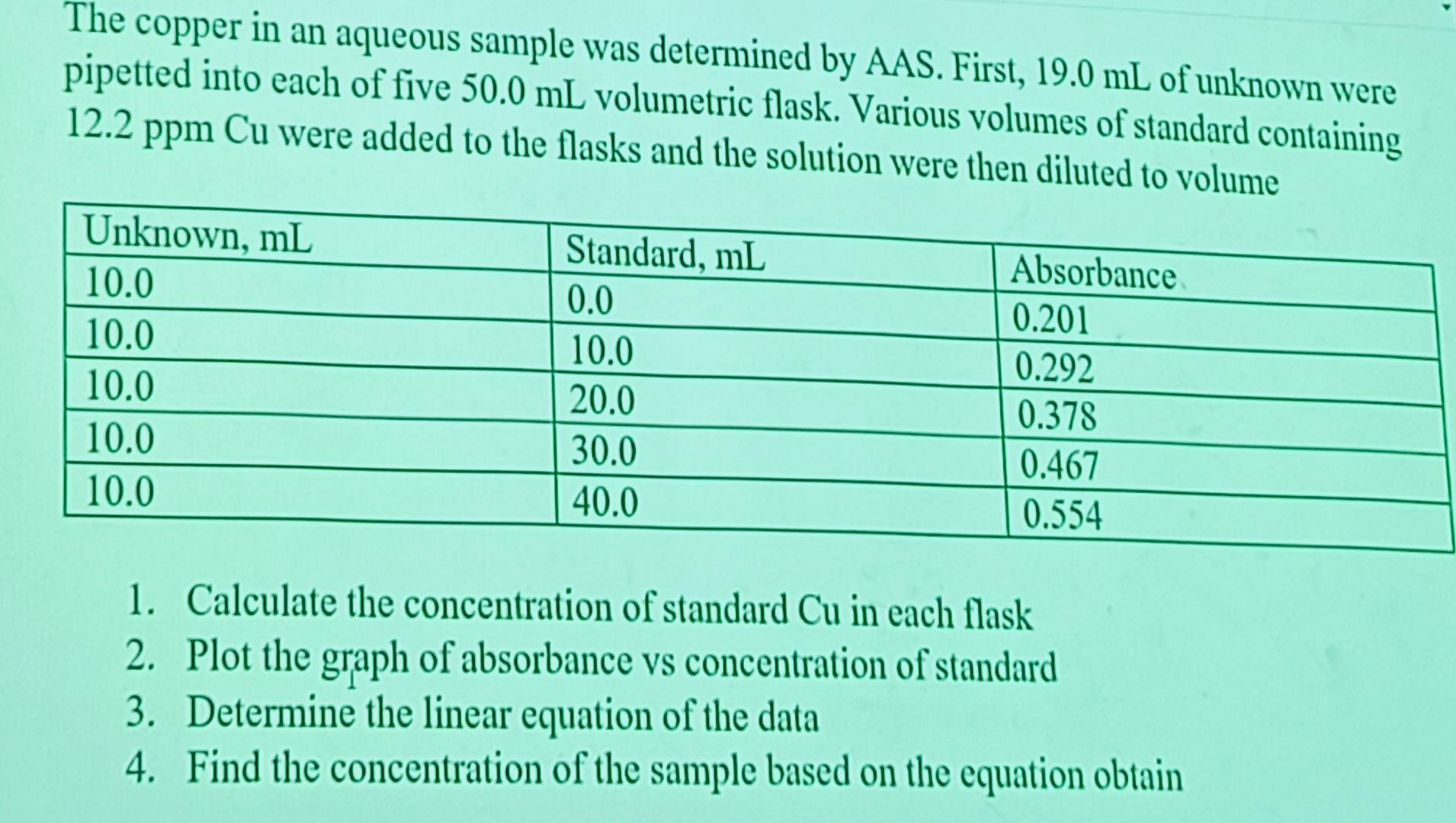
The copper in an aqueous sample was determined by AAS. First, of unknown were pipetted into each of five volumetric flask. Various volumes of standard containing were added to the flasks and the solution were then diluted to volume 1. Calculate the concentration of standard in each flask 2. Plot the graph of absorbance vs concentration of standard 3. Determine the linear equation of the data 4. Find the concentration of the sample based on the equation obtain
Expert Answer
1. Calculating the concentration of standard Cu in each flask: The concentration of standard Cu in each flask can be calculated