Home /
Expert Answers /
Chemistry /
the-concentration-of-urea-ch4n2o-in-a-sample-of-urine-is-9-4lg-the-molar-mass-of-pa796
(Solved): The concentration of urea (CH4N2O) in a sample of urine is 9.4Lg. The molar mass of ...
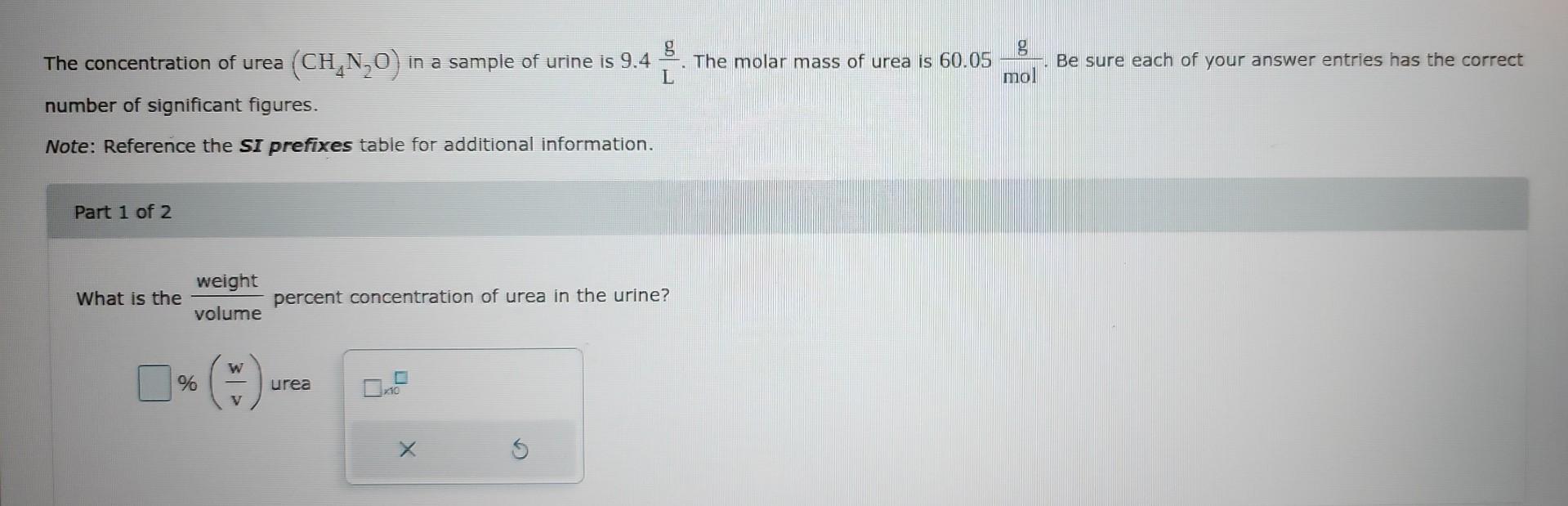
The concentration of urea in a sample of urine is . The molar mass of urea is . Be sure each of your answer entries has the correct number of significant figures. Note: Reference the SI prefixes table for additional information. Part 1 of 2 What is the percent concentration of urea in the urine? urea