Home /
Expert Answers /
Chemistry /
the-concentration-of-mercury-in-canned-tuna-is-determined-using-indium-as-an-internal-standard-pa976
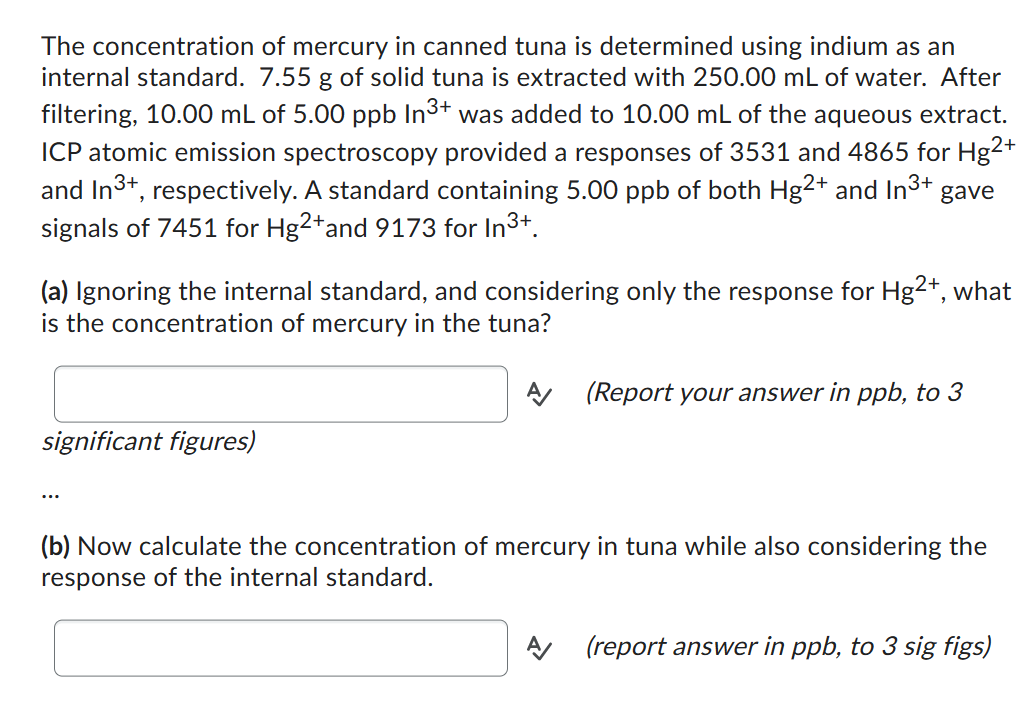
(Solved): The concentration of mercury in canned tuna is determined using indium as an internal standard. \( ...
The concentration of mercury in canned tuna is determined using indium as an internal standard. \( 7.55 \mathrm{~g} \) of solid tuna is extracted with \( 250.00 \mathrm{~mL} \) of water. After filtering, \( 10.00 \mathrm{~mL} \) of \( 5.00 \mathrm{ppb}^{3 \mathrm{n}^{3+}} \) was added to \( 10.00 \mathrm{~mL} \) of the aqueous extract. ICP atomic emission spectroscopy provided a responses of 3531 and 4865 for \( \mathrm{Hg}^{2+} \) and \( \operatorname{In}^{3+} \), respectively. A standard containing \( 5.00 \mathrm{ppb} \) of both \( \mathrm{Hg}^{2+} \) and \( \operatorname{In}^{3+} \) gave signals of 7451 for \( \mathrm{Hg}^{2+} \) and 9173 for \( \ln ^{3+} \). (a) Ignoring the internal standard, and considering only the response for \( \mathrm{Hg}^{2+} \), what is the concentration of mercury in the tuna? A (Report your answer in ppb, to 3 significant figures) (b) Now calculate the concentration of mercury in tuna while also considering the response of the internal standard. A (report answer in ppb, to 3 sig figs)
Expert Answer
(a) To calculate the concentration of mercury in the tuna without considering t