Home /
Expert Answers /
Chemistry /
the-compound-potassium-sulfide-is-a-strong-electrolyte-write-the-reaction-when-solid-potassium-s-pa595
(Solved): The compound potassium sulfide is a strong electrolyte. Write the reaction when solid potassium s ...

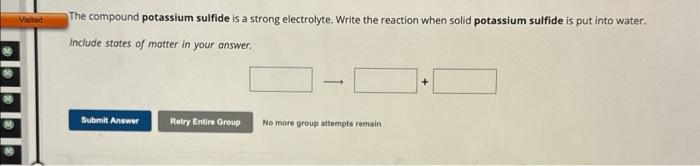
The compound potassium sulfide is a strong electrolyte. Write the reaction when solid potassium sulfide is put into waterInclude stotes of motter in your answer.
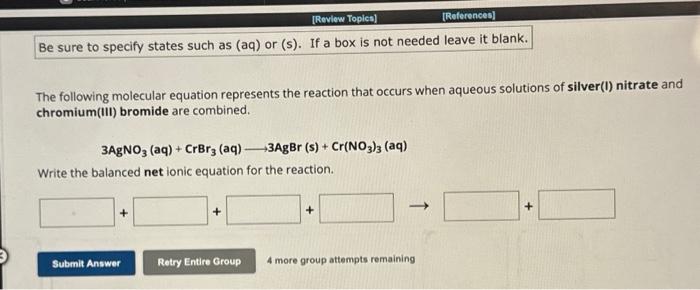
The following molecular equation represents the reaction that occurs when aqueous solutions of silver(I) nitrate and chromium(III) bromide are combined. Write the balanced net ionic equation for the reaction. 4 more group attempts remaining
Use the References to access important values if needed for this question. The compound calcium iodide is a strong electrolyte. Write the reaction when solid calcium iodide is put into water. Include states of matter in your answer.
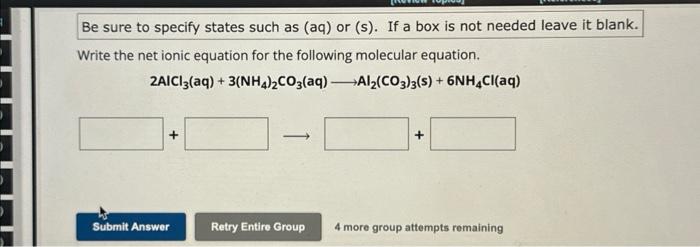
Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. Write the net ionic equation for the following molecular equation. 4 more group attempts remaining