Home /
Expert Answers /
Chemistry /
the-compound-aluminum-trichloride-consists-of-mathrm-al-2-mathrm-cl-5-molecules-with-pa870
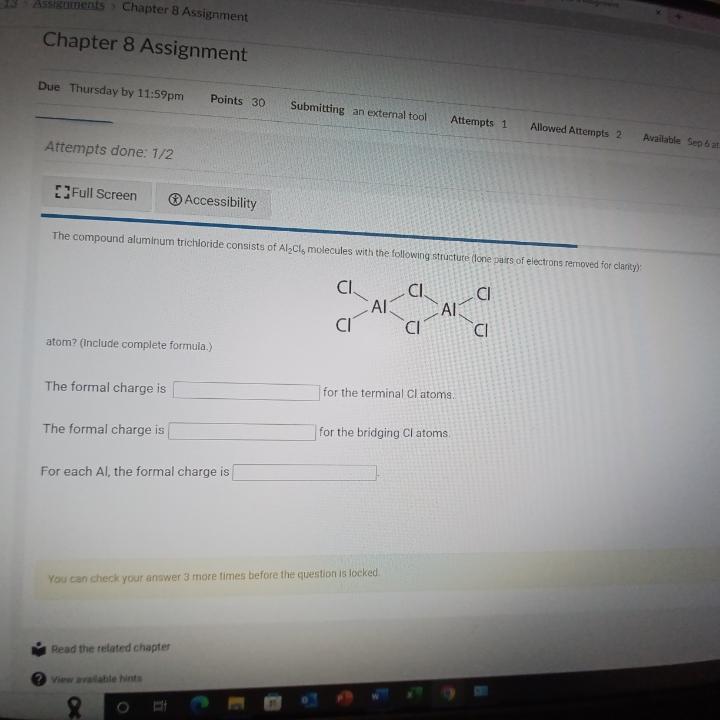
(Solved): The compound aluminum trichloride consists of \( \mathrm{Al}_{2} \mathrm{Cl}_{5} \) molecules with ...
The compound aluminum trichloride consists of \( \mathrm{Al}_{2} \mathrm{Cl}_{5} \) molecules with the following structure done pars of electrons removed for clanty): atom? (include complete formula.) The formal charge is for the terminal Cl atoms. The formal charge is for the bridging \( \mathrm{Cl} \) atoms For each Al, the formal charge is You can check your anawer 3 more times before the question is iocked.
Expert Answer
21 Formal charge of an atom = Number of valence el