Home /
Expert Answers /
Chemistry /
the-combustion-reaction-of-dimethylhydrazine-is-used-to-fuel-rockets-ch-nnh-1-40-g-pa967
(Solved): The combustion reaction of dimethylhydrazine is used to fuel rockets. (CH)NNH (1) + 40(g ...
The combustion reaction of dimethylhydrazine is used to fuel rockets. (CH?)?NNH? (1) + 40?(g) ??? N?(g) + 4H?O(g) +2CO?(g) = -1694 kJ ?? rxn Calculate the enthalpy change for the following reaction: 2N?(g) + 8H?O(g) + 4CO?(g). ? 2(CH²)?NNH? (1) +80?(g) ?? kJ rxn =
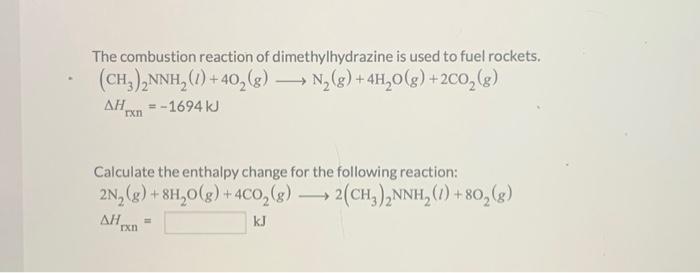
The combustion reaction of dimethylhydrazine is used to fuel rockets. Calculate the enthalpy change for the following reaction: