Home /
Expert Answers /
Chemistry /
the-bromination-of-acetone-is-shown-below-mathrm-ch-3-mathrm-coch-3-mathrm-br-2-pa751
(Solved): The bromination of acetone is shown below... \[ \mathrm{CH}_{3} \mathrm{COCH}_{3}+\mathrm{Br}_{2}+ ...
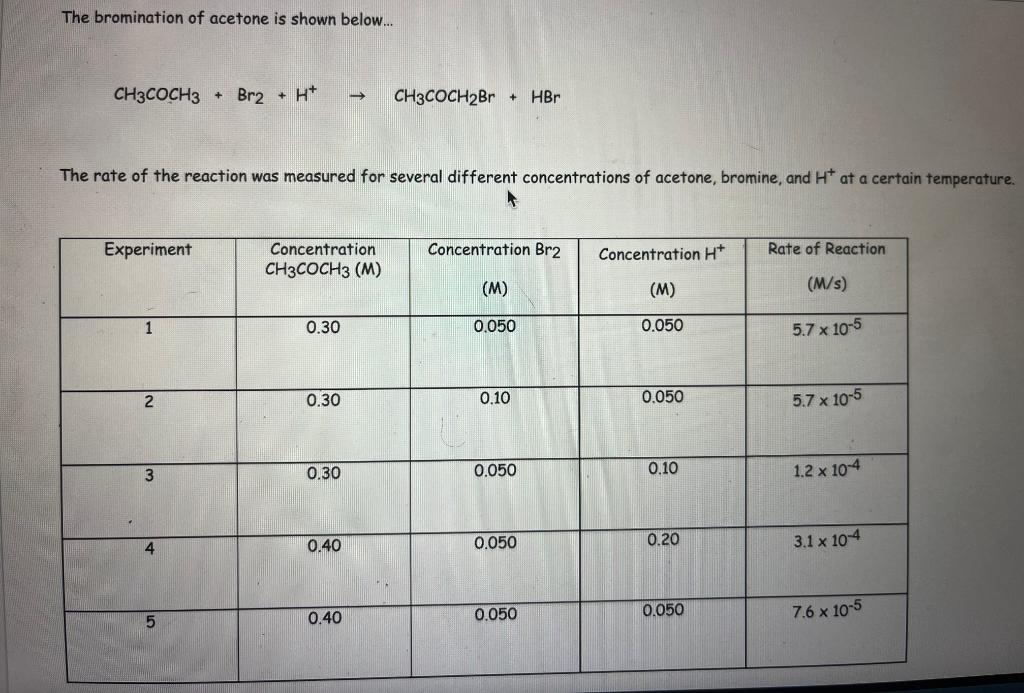
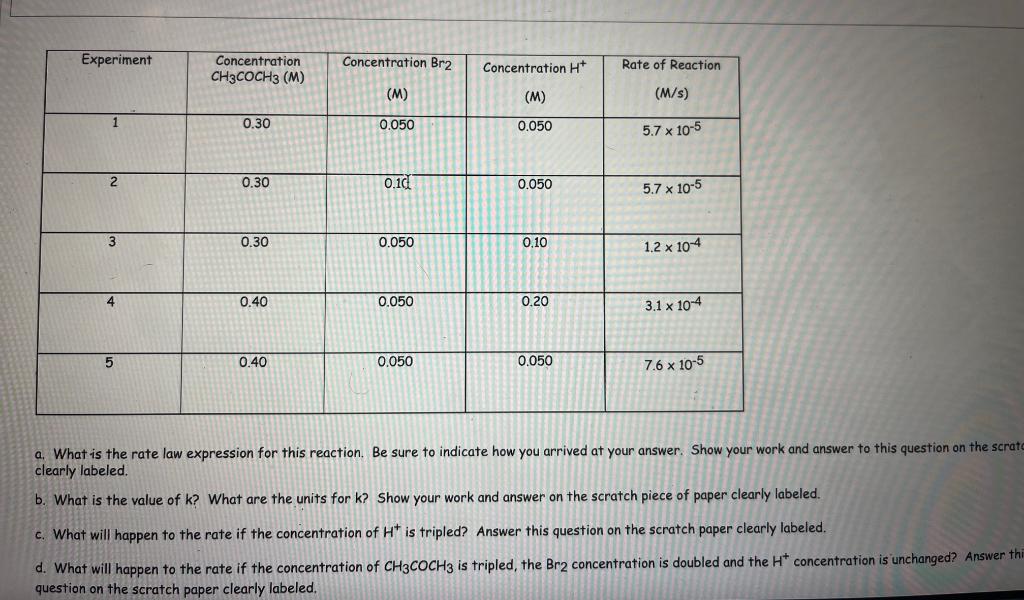
The bromination of acetone is shown below... \[ \mathrm{CH}_{3} \mathrm{COCH}_{3}+\mathrm{Br}_{2}+\mathrm{H}^{+} \rightarrow \mathrm{CH}_{3} \mathrm{COCH}_{2} \mathrm{Br}+\mathrm{HBr} \] The rate of the reaction was measured for several different concentrations of acetone, bromine, and \( \mathrm{H}^{+} \)at a certain temperature
a. What is the rate law expression for this reaction. Be sure to indicate how you arrived at your answer. Show your work and answer to this question on the scrat clearly labeled. b. What is the value of \( k \) ? What are the units for \( k \) ? Show your work and answer on the scratch piece of paper clearly labeled. c. What will happen to the rate if the concentration of \( \mathrm{H}^{+} \)is tripled? Answer this question on the scratch paper clearly labeled. d. What will happen to the rate if the concentration of \( \mathrm{CH}_{3} \mathrm{COCH}_{3} \) is tripled, the \( \mathrm{Br}_{2} \) concentration is doubled and the \( \mathrm{H}^{+} \)concentration is unchanged? Answer th question on the scratch paper clearly labeled.