Home /
Expert Answers /
Chemistry /
the-born-haber-cycle-for-sodium-oxide-is-shown-not-to-scale-3a-calculate-values-for-the-fol-pa407
(Solved): The Born-Haber cycle for sodium oxide is shown (not to scale). 3a. Calculate values for the fol ...
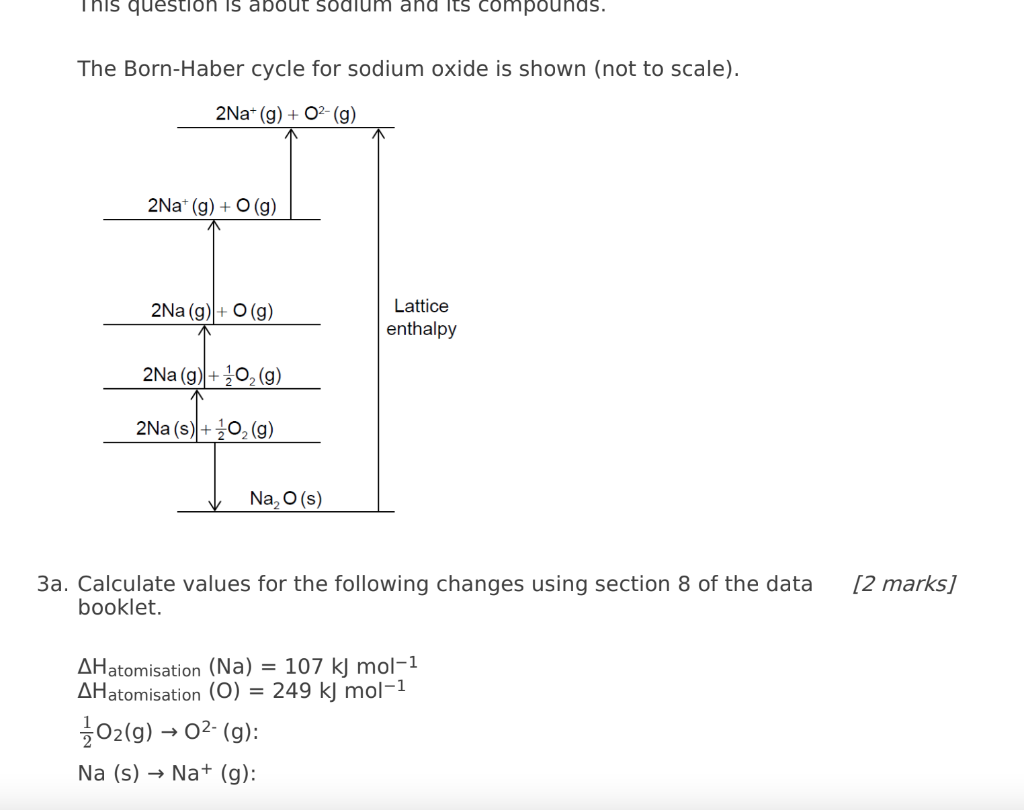
The Born-Haber cycle for sodium oxide is shown (not to scale). 3a. Calculate values for the following changes using section 8 of the data [2 marks] booklet. \( \Delta \mathrm{H}_{\text {atomisation }}(\mathrm{Na})=107 \mathrm{~kJ} \mathrm{\textrm {mol } ^ { - 1 }} \) \( \Delta \mathrm{H}_{\text {atomisation }}(\mathrm{O})=249 \mathrm{~kJ} \mathrm{~mol}^{-1} \) \[ \begin{array}{l} \frac{1}{2} \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{O}^{2-}(\mathrm{g}) \\ \mathrm{Na}(\mathrm{s}) \rightarrow \mathrm{Na}^{+}(\mathrm{g}) \end{array} \]
b. The standard enthalpy of formation of sodium oxide is \( -414 \mathrm{~kJ} \mathrm{~mol}^{-1} \). [2 marks] Determine the lattice enthalpy of sodium oxide, in \( \mathrm{kJ} \mathrm{mol}^{-1} \), using section 8 of the data booklet and your answers to (d)(i). (If you did not get answers to (d)(i), use \( +850 \mathrm{~kJ} \mathrm{~mol}^{-1} \) and \( +600 \mathrm{~kJ} \mathrm{~mol}^{-1} \) respectively, but these are not the correct answers.)
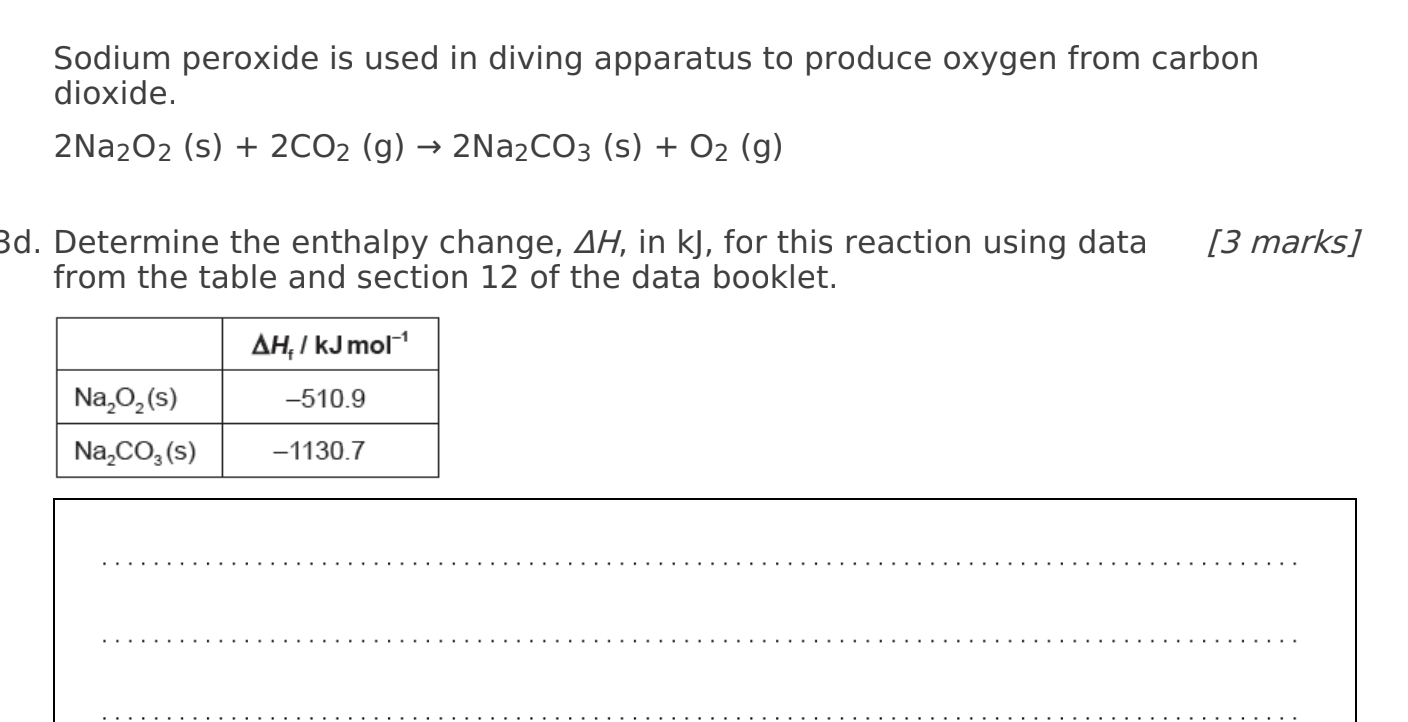
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide. \[ 2 \mathrm{Na}_{2} \mathrm{O}_{2}(\mathrm{~s})+2 \mathrm{CO}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{Na}_{2} \mathrm{CO}_{3}(\mathrm{~s})+\mathrm{O}_{2}(\mathrm{~g}) \] d. Determine the enthalpy change, \( \Delta H \), in \( \mathrm{kJ} \), for this reaction using data from the table and section 12 of the data booklet.
3e. Outline why bond enthalpy values are not valid in calculations such as [1 mark] that in \( (g)(i) \). 3f. An allotrope of molecular oxygen is ozone. Compare, giving a reason, the [1 mark] bond enthalpies of the \( \mathrm{O} \) to \( \mathrm{O} \) bonds in \( \mathrm{O}_{2} \) and \( \mathrm{O}_{3} \).
![b. The standard enthalpy of formation of sodium oxide is \( -414 \mathrm{~kJ} \mathrm{~mol}^{-1} \). [2 marks] Determine the](https://media.cheggcdn.com/media/451/451dd7a5-4ed1-4026-90f7-dcee362e711b/phpK3dk12)
![3e. Outline why bond enthalpy values are not valid in calculations such as
[1 mark] that in \( (g)(i) \).
3f. An allotrope of](https://media.cheggcdn.com/media/d22/d2275912-7a08-4132-a160-87ea02f1c9ec/phphvw65S)