Home /
Expert Answers /
Chemistry /
the-boiling-point-of-liquid-mathrm-b-is-95-5-circ-mathrm-c-at-1-atm-760-0-torr-pa512
(Solved): The boiling point of liquid \( \mathrm{B} \) is \( 95.5^{\circ} \mathrm{C} \) at 1 atm (760.0 torr) ...
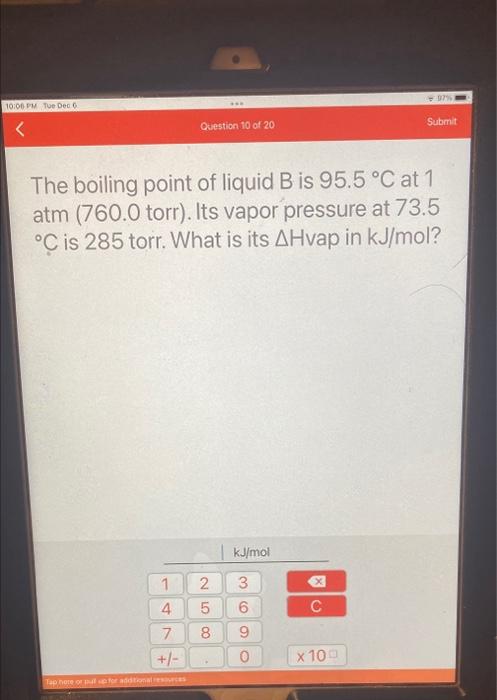
The boiling point of liquid \( \mathrm{B} \) is \( 95.5^{\circ} \mathrm{C} \) at 1 atm (760.0 torr). Its vapor pressure at \( 73.5 \) \( { }^{\circ} \mathrm{C} \) is 285 torr. What is its \( \Delta \) Hvap in \( \mathrm{kJ} / \mathrm{mol} \) ?
Expert Answer
Solution:- Given data :- initial vapor pressure, P1 = 760 torr initial temperature, T1 = 95.5 oC =