Home /
Expert Answers /
Chemistry /
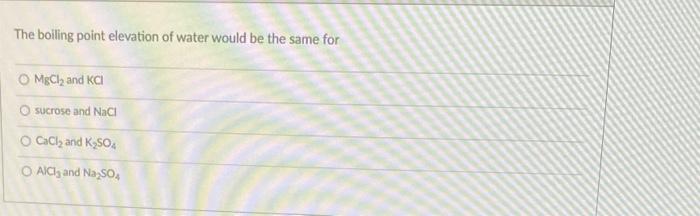
the-boiling-point-elevation-of-water-would-be-the-same-for-mbcl2-and-kcl-sucrose-and-nacl-cacl2-pa686
Expert Answer
Boiling point elevation is one of the Colligative properties. Colligative properties:- It is the properties of the soluti