Home /
Expert Answers /
Chemistry /
the-bohr-hydrogen-atom-model-we-looked-at-this-diagram-for-the-bohr-model-of-the-hydrogen-atom-it-pa491
(Solved): The Bohr Hydrogen Atom model We looked at this diagram for the Bohr model of the hydrogen atom. It ...
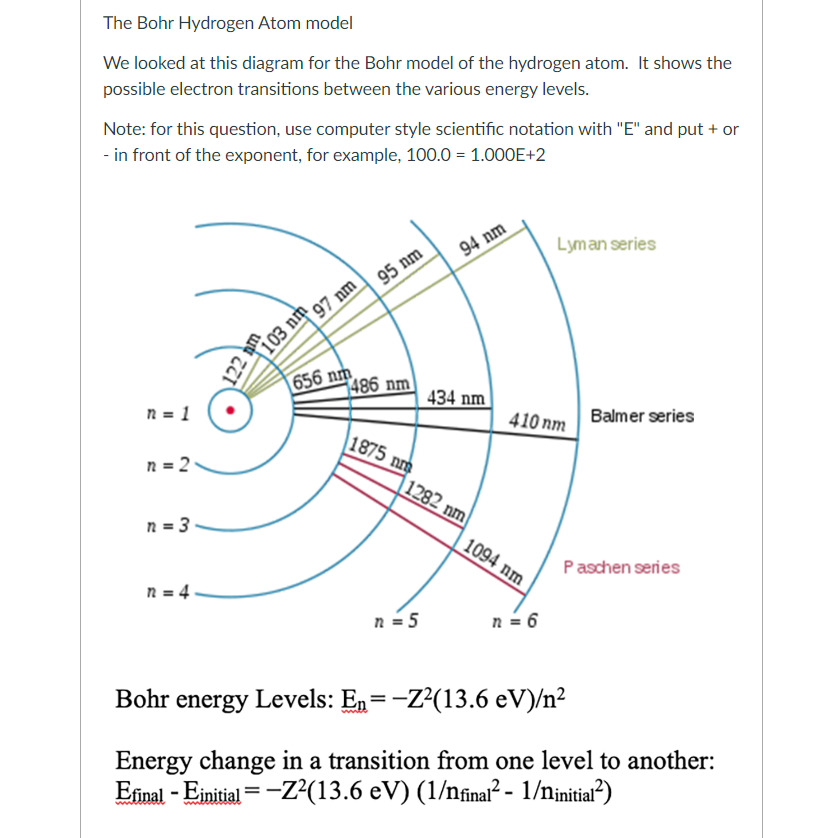
The Bohr Hydrogen Atom model We looked at this diagram for the Bohr model of the hydrogen atom. It shows the possible electron transitions between the various energy levels. Note: for this question, use computer style scientific notation with "E" and put + or - in front of the exponent, for example, \( 100.0=1.000 \mathrm{E}+2 \) Bohr energy Levels: \( \mathrm{E}_{\mathrm{n}}=-Z^{2}(13.6 \mathrm{eV}) / \mathrm{n}^{2} \) Energy change in a transition from one level to another: \( E_{\text {final }}-E_{\text {initial }}=-Z^{2}(13.6 \mathrm{eV})\left(1 / \mathrm{n}_{\text {final }}{ }^{2}-1 / \mathrm{n}_{\text {initial }}{ }^{2}\right) \)
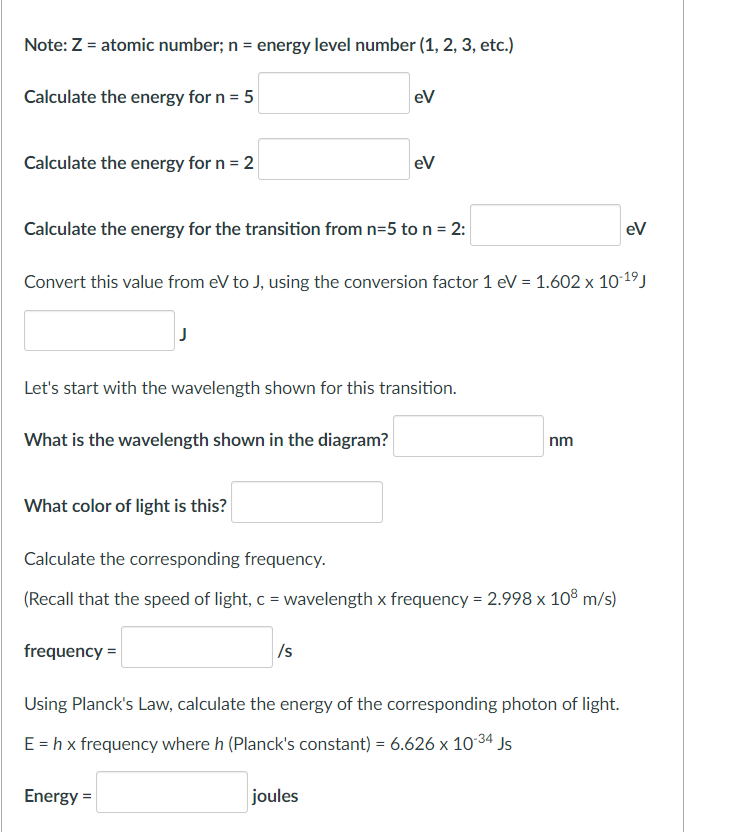
Note: \( Z \) = atomic number; \( n \) = energy level number (1, 2, 3, etc.) Calculate the energy for \( n=5 \) Calculate the energy for \( n=2 \) Calculate the energy for the transition from \( n=5 \) to \( n=2 \) : \( \mathrm{eV} \) Convert this value from eV to J, using the conversion factor \( 1 \mathrm{eV}=1.602 \times 10^{-19} \mathrm{~J} \) J Let's start with the wavelength shown for this transition. What is the wavelength shown in the diagram? \( \mathrm{nm} \) What color of light is this? Calculate the corresponding frequency. (Recall that the speed of light, \( \mathrm{c}= \) wavelength \( \mathrm{x} \) frequency \( =2.998 \times 10^{8} \mathrm{~m} / \mathrm{s} \) ) frequency \( =\mathrm{cm} \) Using Planck's Law, calculate the energy of the corresponding photon of light. \( \mathrm{E}=h \times \) frequency where \( h \) (Planck's constant) \( =6.626 \times 10^{-34} \mathrm{Js} \) Energy = joules
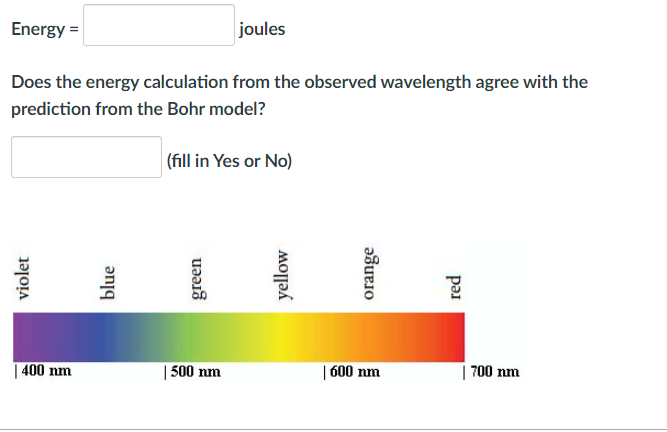
Energy = joules Does the energy calculation from the observed wavelength agree with the prediction from the Bohr model? (fill in Yes or No)