(Solved): The basic process flow diagram is presented in Figure 1. Figure 1: Basic Process Flow Diagram Ethyl ...
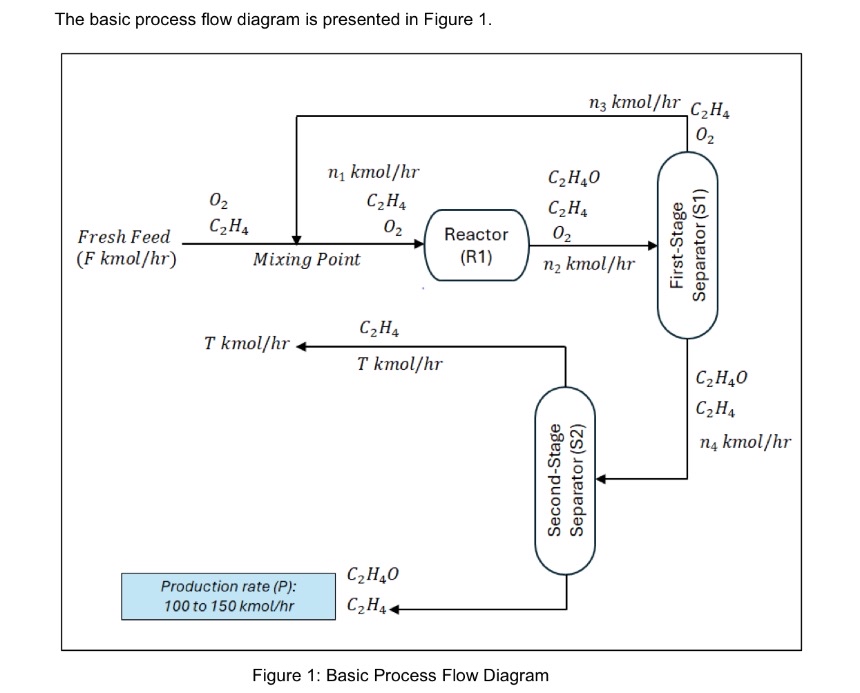
The basic process flow diagram is presented in Figure 1. Figure 1: Basic Process Flow Diagram Ethylene oxide (C2H4O) is used primarily as a sterilizing agent for medical equipment and supplies, and as a chemical intermediate in the production of ethylene glycol and other chemicals. It is produced by the catalytic oxidation of ethylene (C2H4): 2C2H4 +O2 -> 2C2H4O. The feed to the reactor (R1) (not the fresh feed to the process) contains 75 mol% of ethylene and the balance oxygen (O2). The production is designed for 30% of single pass conversion. Product stream exit at R1 enters a two-stage separator unit. The two-stage separator unit is functioned to separate the ethylene oxide (stream exit the R1) from other components. The product stream exit R1 enters the first-stage separator (S1). The S1 top product contains ethylene and oxygen only, while the S1 bottom product consists of ethylene and ethylene oxide only. The top product of S1 is recycled to the reactor. The bottom product of S1 enters second-stage separator (S2). At separator S2, 95% of ethylene fed to the S2 will exit the S2 as top product. Only ethylene present at S2 top product. Meanwhile, S2 bottom product contains 97.7 mol% of ethylene oxide and the balance ethylene. It is noted that the production rate is refer to the molar flowrate of S2 bottom product. Find the F, n1, n2, n3, n4 and T and the molar composition of stream F, n1, n2, n3, n4.