Home /
Expert Answers /
Chemistry /
the-amount-of-heat-q-gained-or-lost-by-a-substance-with-mass-m-as-its-temperature-changes-t-pa584
(Solved): The amount of heat (q) gained or lost by a substance (with mass m ) as its temperature changes (T ...
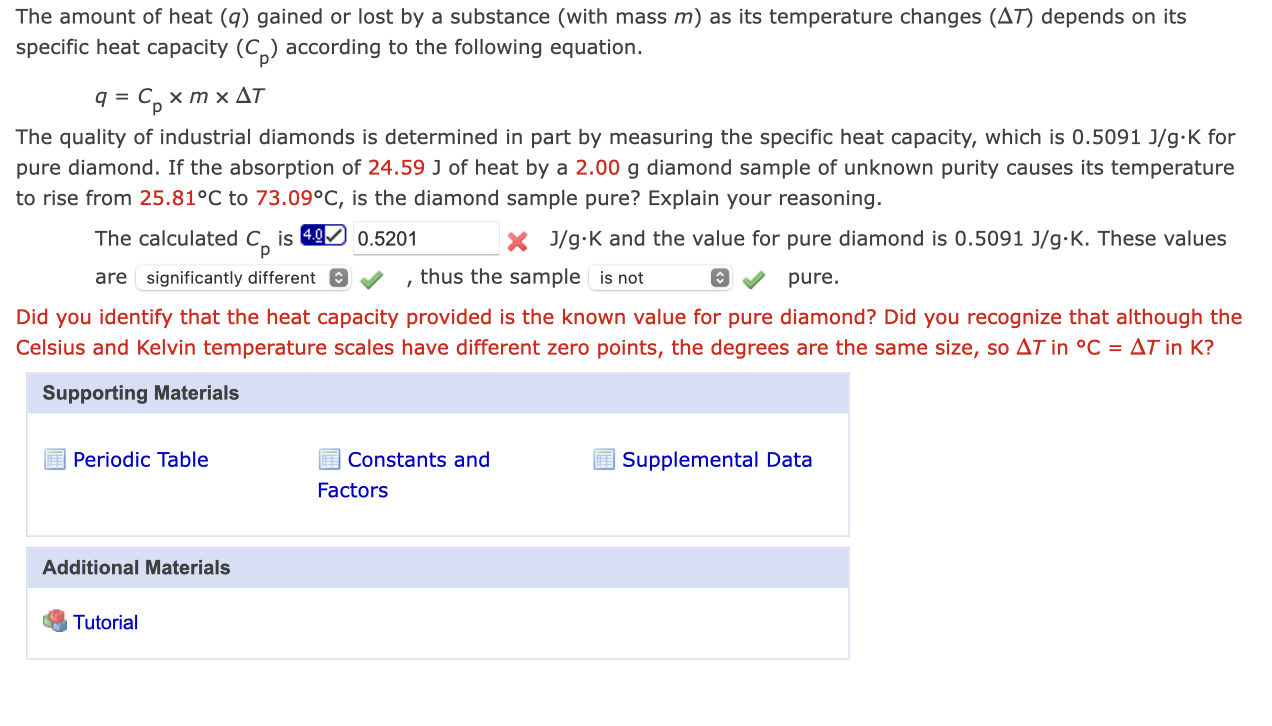
The amount of heat gained or lost by a substance (with mass ) as its temperature changes depends on its specific heat capacity according to the following equation. The quality of industrial diamonds is determined in part by measuring the specific heat capacity, which is for pure diamond. If the absorption of of heat by a diamond sample of unknown purity causes its temperature to rise from to , is the diamond sample pure? Explain your reasoning. The calculated is * and the value for pure diamond is . These values are , thus the sample pure. Did you identify that the heat capacity provided is the known value for pure diamond? Did you recognize that although the Celsius and Kelvin temperature scales have different zero points, the degrees are the same size, so in in ?