Home /
Expert Answers /
Chemistry /
the-airbag-of-a-car-is-filled-when-sodium-azide-mathrm-nan-5-decomposes-to-make-sodium-pa788
(Solved): The airbag of a car is filled when sodium azide, \( \mathrm{NaN}_{5} \), decomposes to make sodium ...
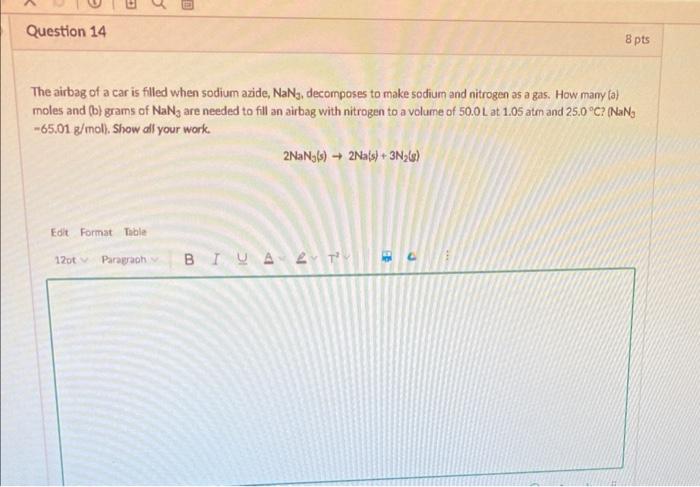
The airbag of a car is filled when sodium azide, \( \mathrm{NaN}_{5} \), decomposes to make sodium and nitrogen as a gas. How many (a) moles and (b) grams of \( \mathrm{NaN}_{3} \) are needed to fill an airbag with nitrogen to a volume of \( 50.0 \mathrm{~L} \) at \( 1.05 \) atm and \( 2.5 .0^{\circ} \mathrm{C} \) ? \( \left(\mathrm{NaN}_{3}\right. \) \( -65.01 \mathrm{~g} / \mathrm{mol} \). Show all your work. \[ 2 \mathrm{NaN}_{j}(s) \rightarrow 2 \mathrm{Na}(s)+3 \mathrm{~N}_{2}(g) \]