Home /
Expert Answers /
Chemistry /
the-activation-energy-of-a-certain-reaction-is-61-0k-j-m-ol-part-a-how-many-times-faster-will-the-pa701
(Solved): The activation energy of a certain reaction is 61.0k(J)/(m)ol. Part A How many times faster will the ...
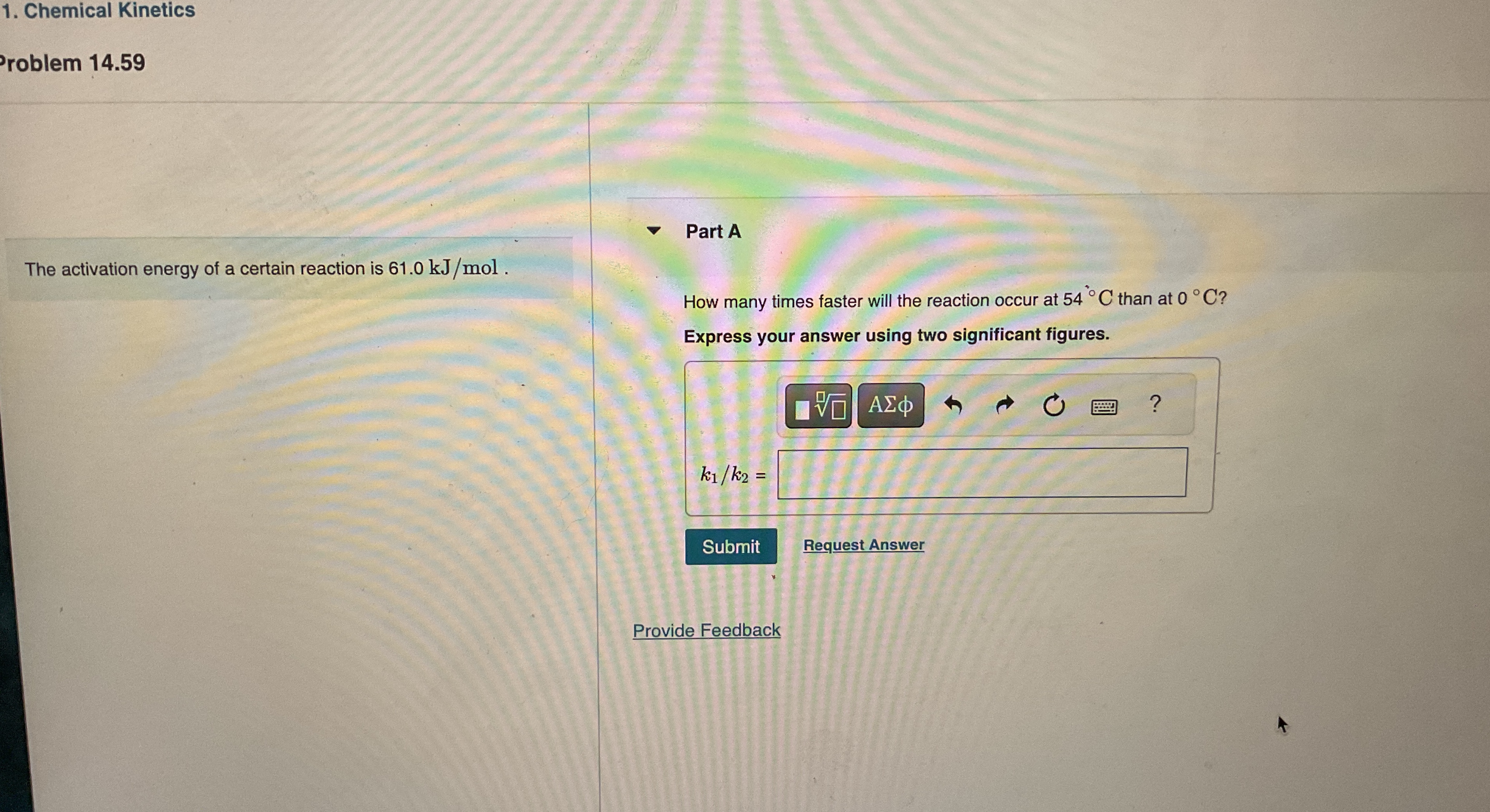
The activation energy of a certain reaction is
61.0k(J)/(m)ol. Part A How many times faster will the reaction occur at
54\deg Cthan at
0\deg C? Express your answer using two significant figures.
(k_(1))/(k_(2))=