Home /
Expert Answers /
Chemistry /
table-1-standard-reduction-potentials-experiment-f-plain-iron-nail-on-the-edge-of-the-petri-d-pa408
(Solved): Table 1: Standard Reduction Potentials Experiment (f): Plain iron nail on the edge of the petri d ...
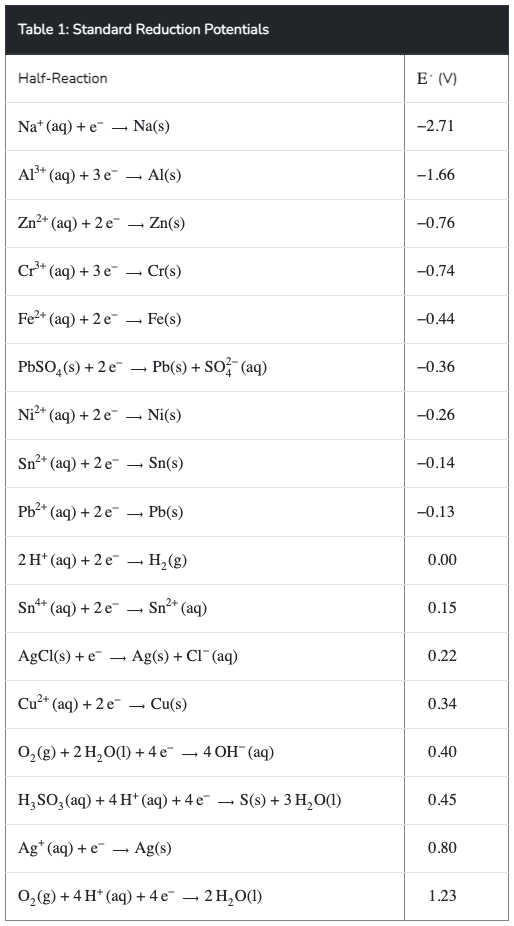
Table 1: Standard Reduction Potentials
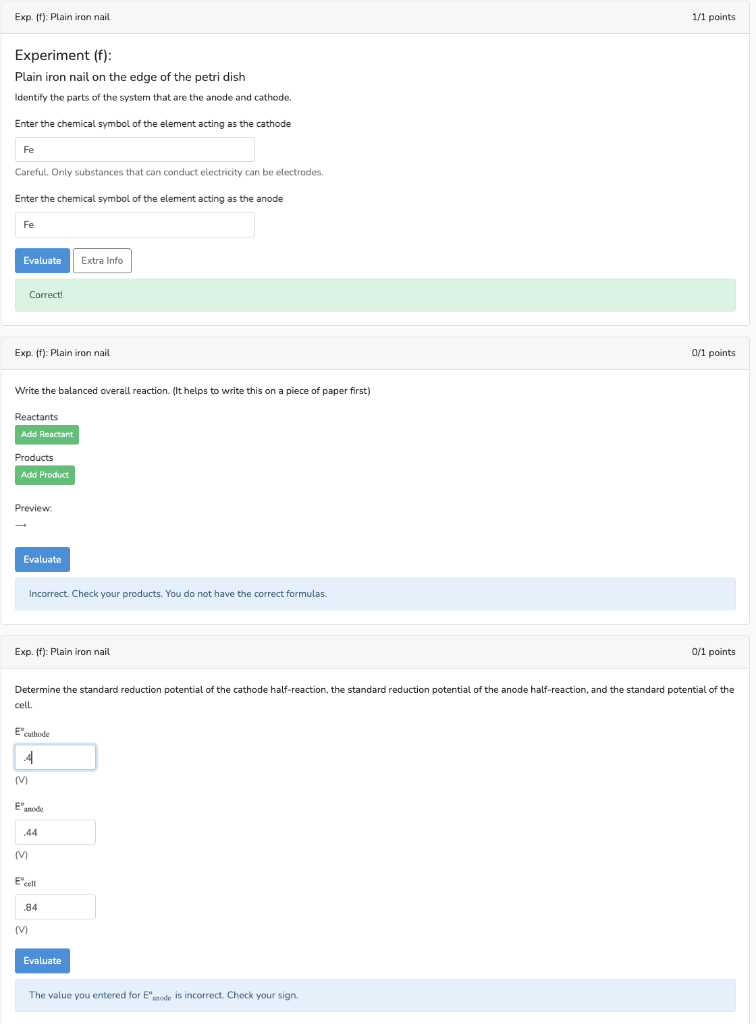
Experiment (f): Plain iron nail on the edge of the petri dish Identify the parts of the system that are the anode and cathode. Enter the chemical symbol of the element acting as the cathode Careful. Only substances that can conduct electricity can be electrodes. Enter the chemical symbol of the element acting as the anode Correct! Exp. (f): Plain iron nail o/1 points Write the balanced overall reaction. (It helps to write this on a piece of paper first) Reactants Products Preview: Incorrect. Check your products. You do not have the correct formulas. Exp. (f): Plain iron nail 0/1 points Determine the standard reduction potential of the cathode half-reaction, the standard reduction potential of the anode half-reaction, and the standard potential of the cell. (V) E (V) (V) The value you entered for is incorrect. Check your sign.