Home /
Expert Answers /
Chemical Engineering /
t-butyl-alcohol-t-was-produced-by-a-gas-phase-reaction-between-water-w-and-isobutene-i-over-a-pa373
(Solved): t-Butyl alcohol (T) was produced by a gas-phase reaction between water (W) and isobutene (I) over a ...
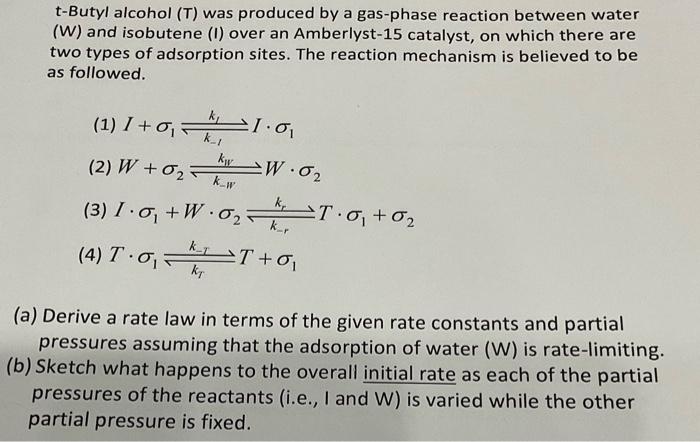
t-Butyl alcohol was produced by a gas-phase reaction between water (W) and isobutene (I) over an Amberlyst-15 catalyst, on which there are two types of adsorption sites. The reaction mechanism is believed to be as followed. (3) (a) Derive a rate law in terms of the given rate constants and partial pressures assuming that the adsorption of water (W) is rate-limiting. (b) Sketch what happens to the overall initial rate as each of the partial pressures of the reactants (i.e., I and ) is varied while the other partial pressure is fixed.