Home /
Expert Answers /
Chemistry /
suppose-the-reaction-between-nitric-oxide-and-bromine-proceeds-by-the-following-mechanism-suppose-pa198
(Solved): Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Suppose ...
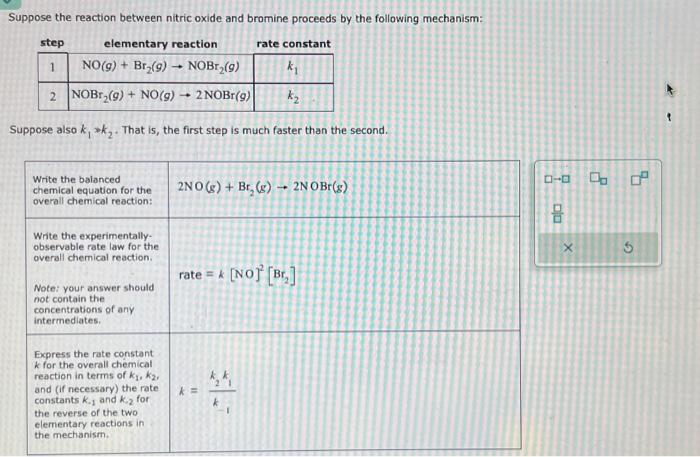
Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Suppose also . That is, the first step is much faster than the second. \begin{tabular}{l} \begin{tabular}{l} Write the balanced \\ chemical equation for the \\ overall chemical reaction: \end{tabular} \\ \begin{tabular}{l} Write the experimentally. \\ observable rate law for the \\ overall chemical reaction. \end{tabular} \\ \begin{tabular}{l} Note: your answer should \\ not contain the \\ concentrations of any \\ intermediates. \end{tabular} \\ \begin{tabular}{l} Express the rate constant \\ for the overall chemical \\ reaction in terms of , \\ and (if necessary) the rate \\ constants and for \\ the reverse of the two \\ elementary reactions in \\ the mectianism. \end{tabular} \\ \hline \end{tabular}