Home /
Expert Answers /
Chemistry /
suppose-that-you-have-a-gas-confined-to-a-cylinder-with-a-movable-piston-determine-how-you-would-pa372
(Solved): Suppose that you have a gas confined to a cylinder with a movable piston. Determine how you would ...
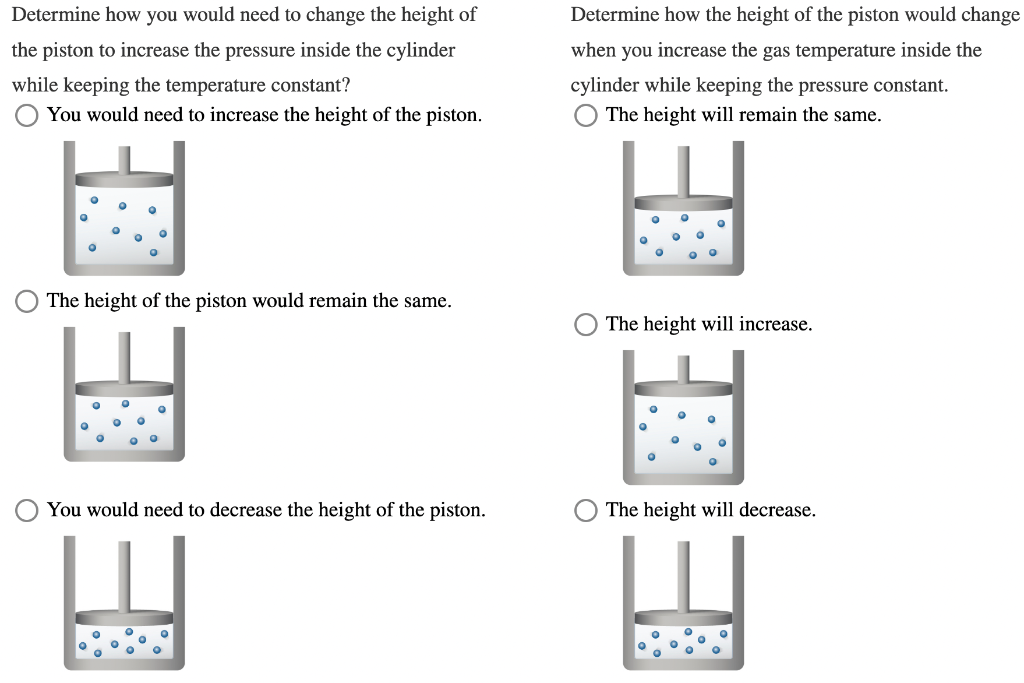
Suppose that you have a gas confined to a cylinder with a movable piston.
Determine how you would need to change the height of Determine how the height of the piston would change the piston to increase the pressure inside the cylinder when you increase the gas temperature inside the while keeping the temperature constant? cylinder while keeping the pressure constant. You would need to increase the height of the piston. The height will remain the same. The height of the piston would remain the same. The height will increase. You would need to decrease the height of the piston. The height will decrease.