Home /
Expert Answers /
Chemistry /
suppose-0-100-mathrm-mol-of-a-perfect-gas-having-mathrm-c-mathrm-v-mathrm-m-pa991
(Solved): Suppose \( 0.100 \mathrm{~mol} \) of a perfect gas having \( \mathrm{C}_{\mathrm{V}, \mathrm{m}}= \ ...
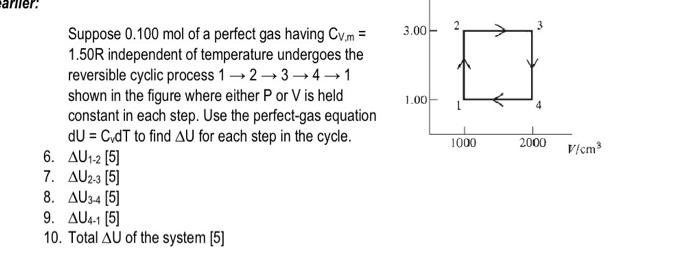
Suppose \( 0.100 \mathrm{~mol} \) of a perfect gas having \( \mathrm{C}_{\mathrm{V}, \mathrm{m}}= \) \( 1.50 \mathrm{R} \) independent of temperature undergoes the reversible cyclic process \( 1 \rightarrow 2 \rightarrow 3 \rightarrow 4 \rightarrow 1 \) shown in the figure where either \( \mathrm{P} \) or \( \mathrm{V} \) is held constant in each step. Use the perfect-gas equation \( \mathrm{dU}=\mathrm{C} v \mathrm{dT} \) to find \( \Delta \mathrm{U} \) for each step in the cycle. 6. \( \Delta \bigcup_{1-2}[5] \) 7. \( \Delta \mathrm{U}_{2-3}[5] \) 8. \( \Delta \mathrm{U}_{3-4}[5] \) 9. \( \Delta \mathrm{U}_{4-1}[5] \) 10. Total \( \Delta \mathrm{U} \) of the system [5]