Home /
Expert Answers /
Chemistry /
sulfate-in-soil-on-mars-in-2008-professor-sam-kounaves-and-his-students-at-tufts-university-had-pa446
(Solved): Sulfate in soil on Mars. In 2008, Professor Sam Kounaves and his students at Tufts University had ...
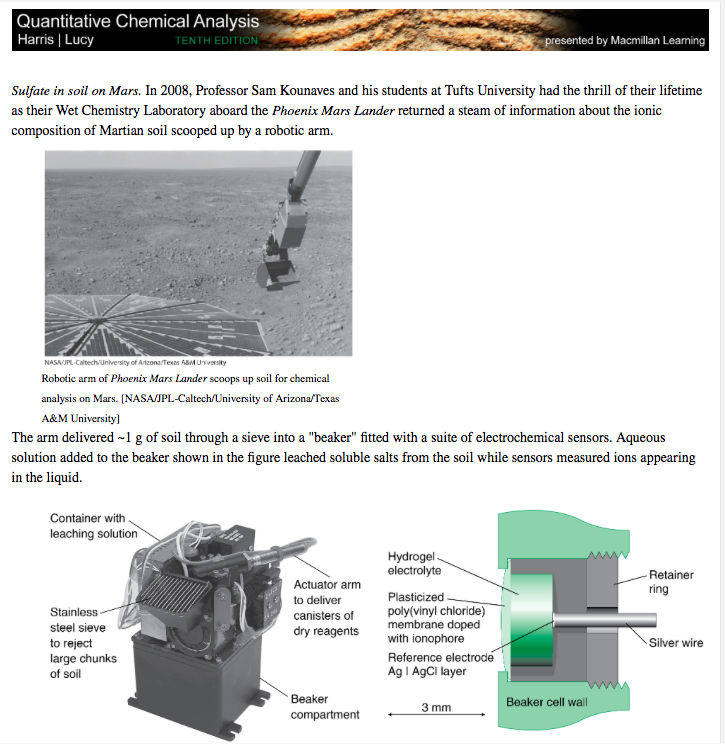
Sulfate in soil on Mars. In 2008, Professor Sam Kounaves and his students at Tufts University had the thrill of their lifetime as their Wet Chemistry Laboratory aboard the Phoenix Mars Lander returned a steam of information about the ionic composition of Martian soil scooped up by a robotic arm. Robotic arm of Phoenix Mars Lander scoops up soil for chemical analysis on Mars. [NASA/JPL-Caltech/University of Arizona/Texas A\&M University] The arm delivered \( \sim 1 \mathrm{~g} \) of soil through a sieve into a "beaker" fitted with a suite of electrochemical sensors. Aqueous solution added to the beaker shown in the figure leached soluble salts from the soil while sensors measured ions appearing in the liquid.
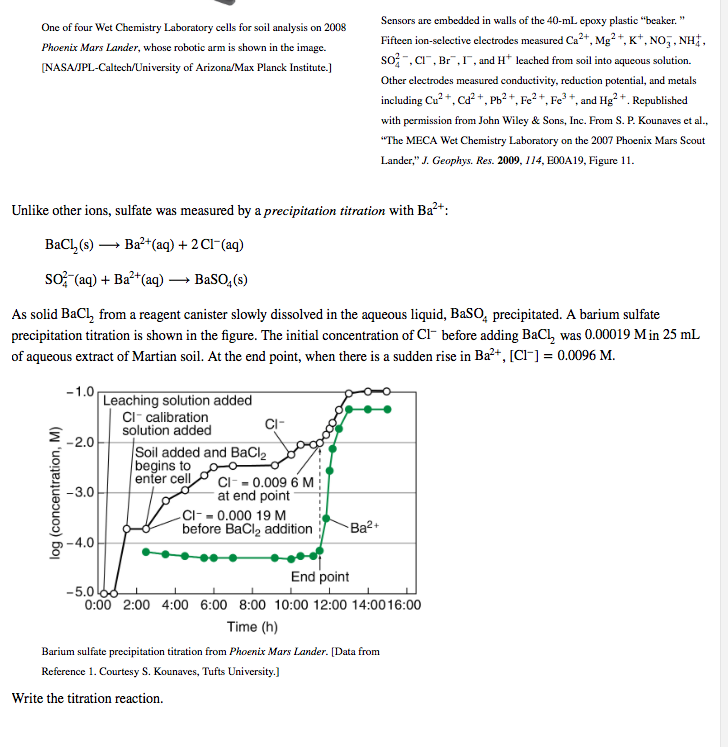
One of four Wet Chemistry Laboratory cells for soil analysis on \( 2008 \quad \) Sensors are embedded in walls of the 40 -mL epoxy plastic "beaker. " Phoenix Mars Lander, whose robotic arm is shown in the image. Fifteen ion-selective electrodes measured \( \mathrm{Ca}^{2+} \), \( \mathrm{Mg}^{2+} \), \( \mathrm{K}^{+}, \mathrm{NO}_{3}^{-}, \mathrm{NH}_{4}^{+} \), [NASA/JPL-Caltech/University of Arizona/Max Planck Institute.] \( \mathrm{SO}_{4}^{2}, \mathrm{Cl}^{-}, \mathrm{Br}^{-}, \mathrm{I}^{-} \), and \( \mathrm{H}^{+} \)leached from soil into aqueous solution. Other electrodes measured conductivity, reduction potential, and metals including \( \mathrm{Cu}^{2+}, \mathrm{Cd}^{2+}, \mathrm{Pb}^{2+}, \mathrm{Fe}^{2}+, \mathrm{Fe}^{3+} \), and \( \mathrm{Hg}^{2}+ \). Republished with permission from John Wiley \& Sons, Inc. From S. P. Kounaves et al., "The MECA Wet Chemistry Laboratory on the 2007 Phoenix Mars Scout Lander," J. Geophys. Res. 2009, I14, E00A19, Figure \( 11 . \) Unlike other ions, sulfate was measured by a precipitation titration with \( \mathrm{Ba}^{2+} \) : \[ \begin{array}{l} \mathrm{BaCl}_{2}(\mathrm{~s}) \longrightarrow \mathrm{Ba}^{2+}(\mathrm{aq})+2 \mathrm{Cl}^{-}(\mathrm{aq}) \\ \mathrm{SO}_{4}^{2-}(\mathrm{aq})+\mathrm{Ba}^{2+}(\mathrm{aq}) \longrightarrow \mathrm{BaSO}_{4}(\mathrm{~s}) \end{array} \] As solid \( \mathrm{BaCl}_{2} \) from a reagent canister slowly dissolved in the aqueous liquid, \( \mathrm{BaSO}_{4} \) precipitated. \( \mathrm{A} \) barium sulfate precipitation titration is shown in the figure. The initial concentration of \( \mathrm{Cl}^{-} \)before adding \( \mathrm{BaCl}_{2} \) was \( 0.00019 \mathrm{M} \) in \( 25 \mathrm{~mL} \) of aqueous extract of Martian soil. At the end point, when there is a sudden rise in \( \mathrm{Ba}^{2+},\left[\mathrm{Cl}^{-}\right]=0.0096 \mathrm{M} \). Barium sulfate precipitation titration from Phoenix Mars Lander. [Data from Reference 1. Courtesy S. Kounaves, Tufts University.] Write the titration reaction.
Write the titration reaction. titration reaction: How many mmol of \( \mathrm{BaCl}_{2} \) were required to reach the end point? millimoles of \( \mathrm{BaCl}_{2} \) : How many mmol of \( \mathrm{SO}_{4}^{2-} \) were contained in the \( 25 \mathrm{~mL} \) ? millimoles of \( \mathrm{SO}_{4}^{2-} \) : If \( \mathrm{SO}_{4}^{2-} \) is derived from \( 0.961 \mathrm{~g} \) of soil, what is the \( \mathrm{wt} \% \) of \( \mathrm{SO}_{4}^{2-} \) in the soil? weight percent of \( \mathrm{SO}_{4}^{2-} \) : 1. S. P. Kounaves, M. H. Hecht, J. Kapit, R. C. Quinn, D. C. Catling, B. C. Clark, D. W. Ming, K. Godpodinova, P. Hredzak, K. McElhoney, and J. Shusterman, Geophys. Res. Lett. 2010, 37, L09201.
Expert Answer
-Reaction of titration: Ba2+ (aq) + SO42- (aq) BaSO4 (s) -Calculation of the mmoles of Ba2+: a) calculation of the change of [Cl-] during the titration: MCl=(0.0096 - 0.00