Home /
Expert Answers /
Chemistry /
study-this-chemical-reaction-mathrm-feso-4-a-q-mathrm-mg-mathrm-s-rightarrow-mathrm-pa346
(Solved): Study this chemical reaction: \[ \mathrm{FeSO}_{4}(a q)+\mathrm{Mg}(\mathrm{s}) \rightarrow \mathrm ...
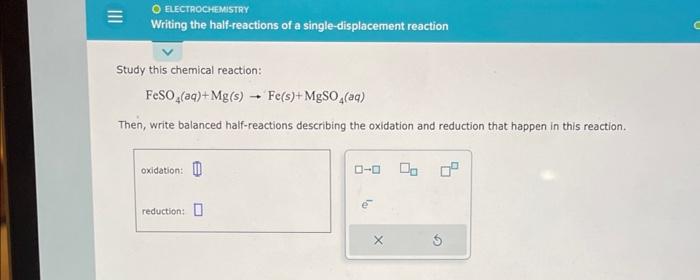
Study this chemical reaction: \[ \mathrm{FeSO}_{4}(a q)+\mathrm{Mg}(\mathrm{s}) \rightarrow \mathrm{Fe}(s)+\mathrm{MgSO}_{4}(a q) \] Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
Expert Answer
The balanced half reactions of the following single-displacement redox reaction FeSO4(aq) +