Home /
Expert Answers /
Chemistry /
study-the-chemical-equations-in-the-table-equation-number-balanced-equation1-2al-s-3cl2-g-2al3-pa835
(Solved): Study the chemical equations in the table.Equation Number Balanced Equation1 2Al(s)+3Cl2(g)2Al3+ ...
Study the chemical equations in the table.
Equation Number Balanced Equation
1 2Al(s)+3Cl2(g)?2Al3+(aq)+6Cl?(aq)
2 HCl(g)+NH3(g)?NH4Cl(s)
3 10H+(aq)+SO2?4(aq)+8I?(aq)?4I2(s)+H2S(g)+4H2O(l)
Classify each reactant in the chemical equations as an oxidizing agent, a reducing agent, or neither.
Oxidizing agent
Reducing agent
Neither
Calculate the increase or decrease in the oxidation state for each element listed as it changes from a reactant to a product. Use a negative sign to show a decrease in oxidation state.
aluminum, beginning in the reactant Al
nitrogen, beginning in the reactant NH3
iodine, beginning in the reactant I?
sulfur, beginning in the reactant SO2?4
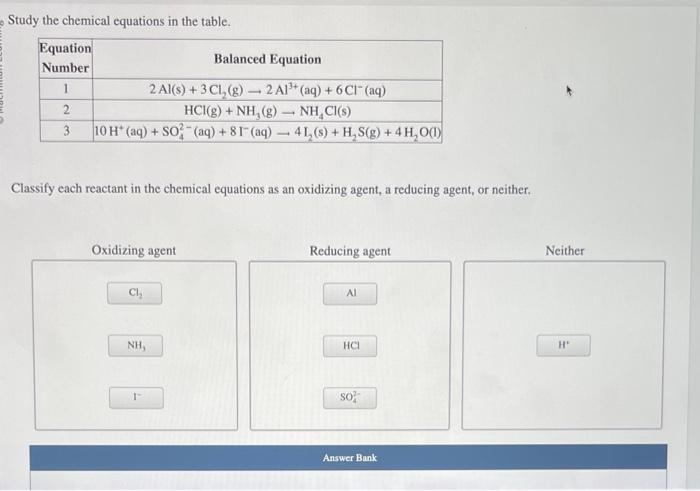

Study the chemical equations in the table. Classify each reactant in the chemical equations as an oxidizing agent, a reducing agent, or neither.
Calculate the increase or decrease in the oxidation state for cach element listed as it changes from a reactant to a product. Use a negative sign to show a decrease in oxidation state. aluminum, beginning in the reactant \( \mathrm{Al} \) nitrogen, beginning in the reactant \( \mathrm{NH}_{3} \) iodine, beginning in the reactant \( \mathrm{I}^{-} \) sulfur, beginning in the reactant \( \mathrm{SO}_{4}^{2-} \)