Home /
Expert Answers /
Chemistry /
stoichiometry-and-yields-of-a-decomposition-reaction-ammonium-dichromate-decomposes-according-to-t-pa935
(Solved): Stoichiometry and Yields of a Decomposition Reaction Ammonium dichromate decomposes according to t ...
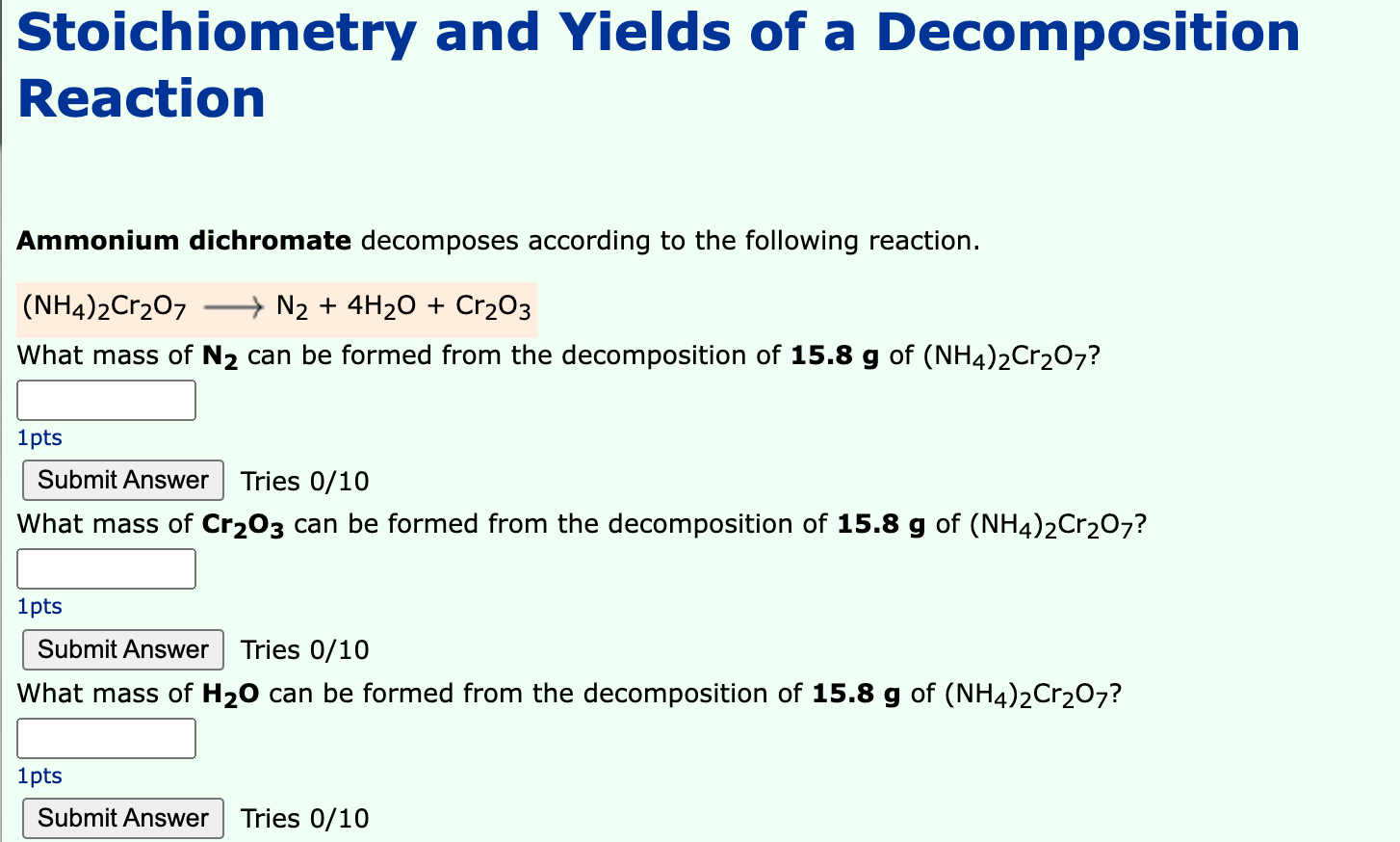
Stoichiometry and Yields of a Decomposition Reaction Ammonium dichromate decomposes according to the following reaction. (NH4)2Cr?O7 ? N? + 4H?O + Cr?O3 What mass of N? can be formed from the decomposition of 15.8 g of (NH4)2Cr?O7? 1pts Submit Answer Tries 0/10 What mass of Cr?03 can be formed from the decomposition of 15.8 g of (NH4)2Cr?O7? 1pts Submit Answer Tries 0/10 What mass of H?O can be formed from the decomposition of 15.8 g of (NH4)2Cr?O7? 1pts Submit Answer Tries 0/10
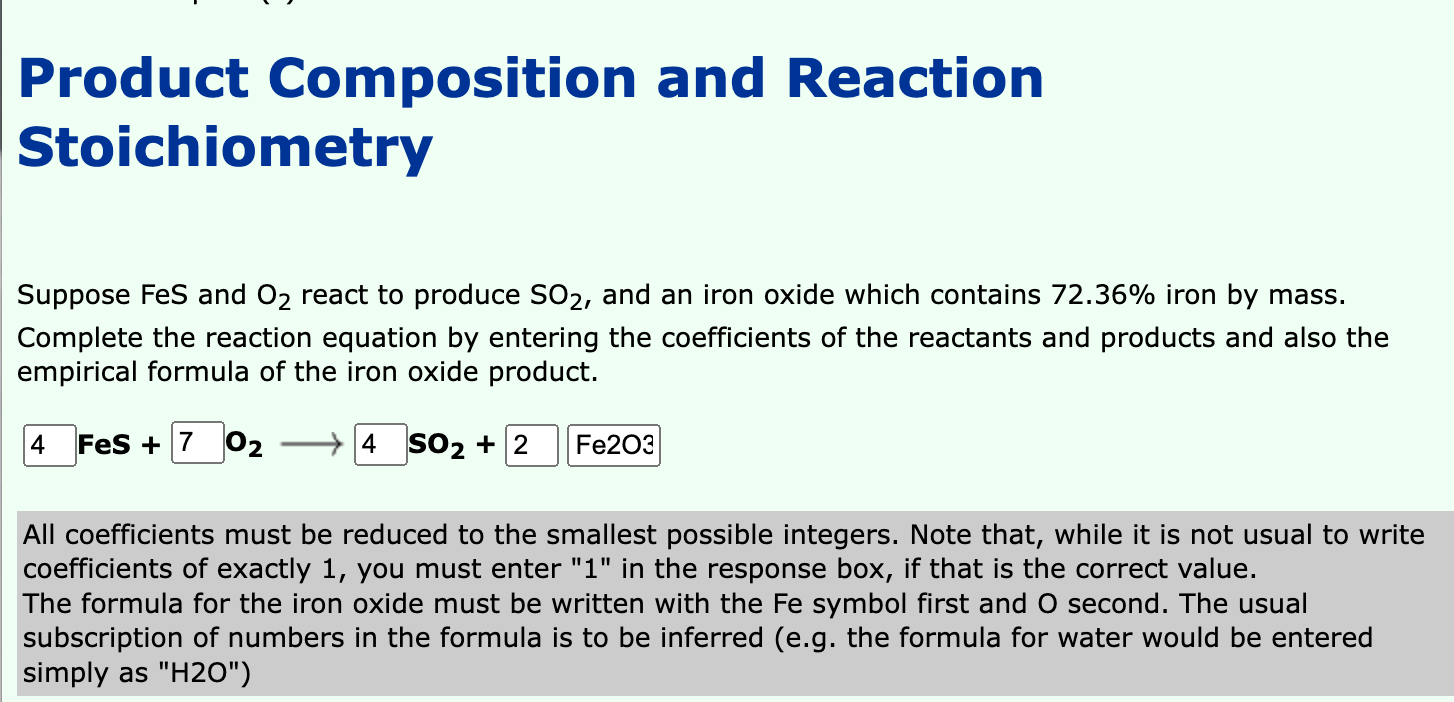
Product Composition and Reaction Stoichiometry Suppose FeS and O? react to produce SO?, and an iron oxide which contains 72.36% iron by mass. Complete the reaction equation by entering the coefficients of the reactants and products and also the empirical formula of the iron oxide product. 4 FeS + 7 02 4 SO? + 2 Fe203 All coefficients must be reduced to the smallest possible integers. Note that, while it is not usual to write coefficients of exactly 1, you must enter "1" in the response box, if that is the correct value. The formula for the iron oxide must be written with the Fe symbol first and O second. The usual subscription of numbers in the formula is to be inferred (e.g. the formula for water would be entered simply as "H20")