Home /
Expert Answers /
Chemistry /
standardization-of-prepared-edta-solution-with-mathrm-zn-2-solution-titration-of-hard-wate-pa882
(Solved): Standardization of prepared EDTA solution with \( \mathrm{Zn}+2 \) solution Titration of hard wate ...
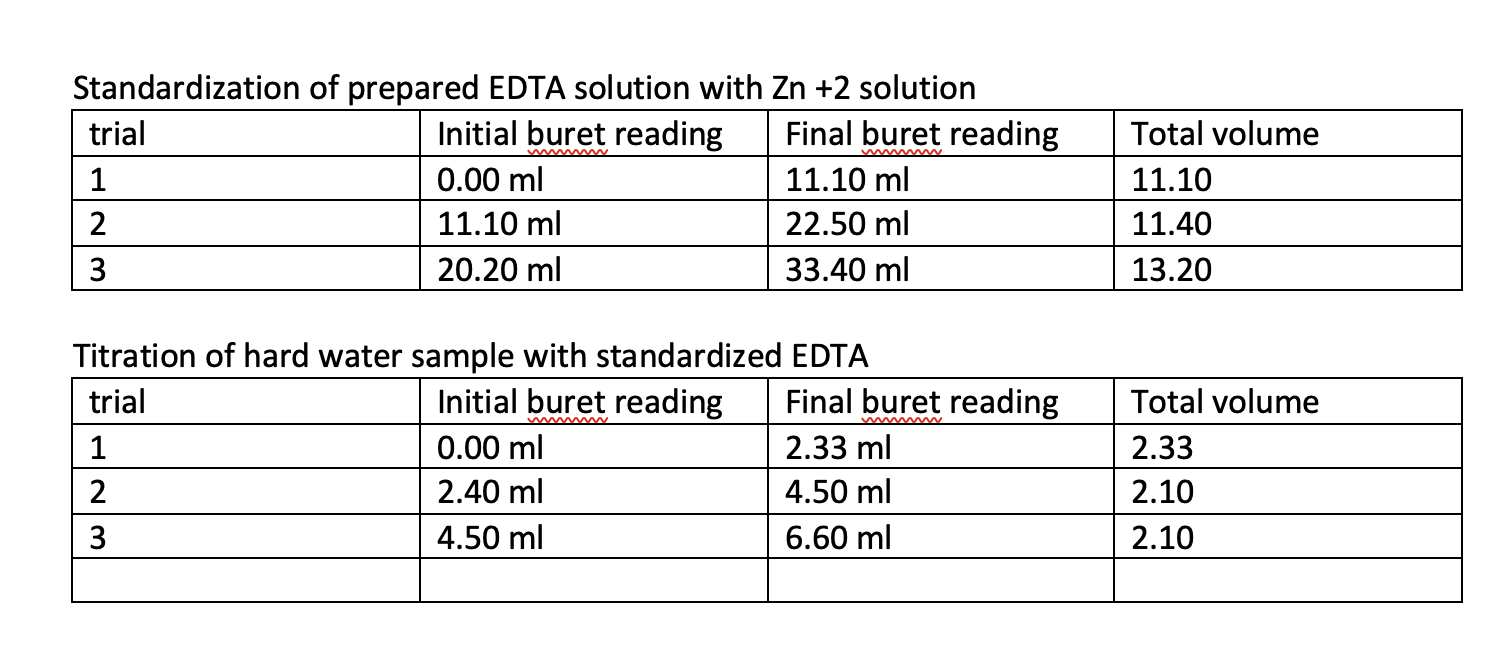
Standardization of prepared EDTA solution with \( \mathrm{Zn}+2 \) solution Titration of hard water sample with standardized EDTA
1. Determine the molarity of your EDTA solution. Since \( \mathrm{Zn}^{2+} \) reacts with EDTA in a 1:1 mole ratio the moles of EDTA will be equal to the moles of \( \mathrm{Zn}^{2+} \). For a solution, moles can be determined by multiplying molarity by volume. \( \mathrm{mol} \mathrm{EDTA}=\operatorname{mol~Zn}^{2+} \) \( \mathrm{M}_{\mathrm{EDTA}} \mathrm{V}_{\mathrm{EDTA}}=\mathrm{M}_{\mathrm{Zn} 2+} \mathrm{V}_{\mathrm{Zn} 2+} \) rearranging the equation above we get: \( \mathrm{M}_{\mathrm{EDTA}}=\mathrm{M}_{\mathrm{Zn} 2+} \mathrm{V}_{\mathrm{Zn} 2+} / \mathrm{V}_{\mathrm{EDTA}} \) 2. Determine an average value and a standard deviation for your EDTA molarity. You can do this on your calculator or use excel. 3. Calculate the value for total water hardness (amount of \( \mathrm{Ca}+\mathrm{Mg} \) ). Following convention, this will be reported in terms of \( \mathrm{CaCO}_{3} \) in units of \( \mathrm{mg} / \mathrm{L} \). The moles of hard water is equal to the moles of EDTA used in the titration. Use the equation \( \mathrm{M}_{\mathrm{EDTA}} \mathrm{V}_{\mathrm{EDTA}}= \) \( M_{\text {hardwater }} V_{\text {hardwater to solve for the molarity of the hard water and then convert the molarity }} \) into units of \( \mathrm{mg} / \mathrm{L} \) using the molar mass of \( \mathrm{CaCO}_{3} \) which is \( 100 \mathrm{~g} \) and the conversion factor \( 1000 \mathrm{mg}=1.00 \mathrm{~g} \). For the molarity of the EDTA, use your average value as determined in step 2 above. The equation is as follows: 4. Calculate the magnesium hardness (reported in terms of \( \mathrm{CaCO}_{3} \) in units of \( \mathrm{mg} / \mathrm{L} \), just like the total hardness). Use the equation that follows. magnesium hardness \( = \) total hardness(from step 3) \( - \) calcium hardness(given by your instructor)