Home /
Expert Answers /
Chemical Engineering /
soru-6-3-a-b-longrightarrow-the-2-c-reaction-is-carried-out-in-pa846
(Solved): Soru-6: \( 3 A+B \longrightarrow \) The \( 2 C \) reaction is carried out in ...
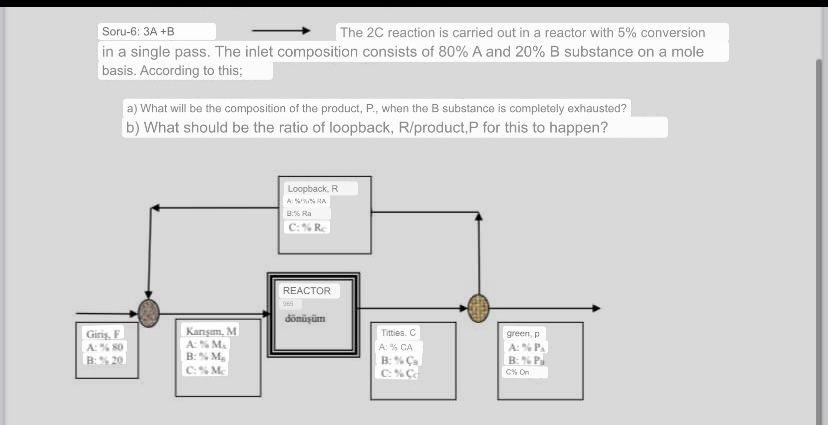
Soru-6: \( 3 A+B \longrightarrow \) The \( 2 C \) reaction is carried out in a reactor with \( 5 \% \) conversion in a single pass. The inlet composition consists of \( 80 \% \) A and \( 20 \% \) B substance on a mole basis. According to this: a) What will be the composition of the product, P., when the B substance is completely exhausted? b) What should be the ratio of loopback, R/product,P for this to happen?
Expert Answer
a) When the B substance is completely exhausted, the composition of the product P will be 80% A and 20% C. This is because the inlet composition consists of 80% A and 20% B, a