Home /
Expert Answers /
Chemistry /
some-vapor-pressures-of-liquid-hg-are-equilibrium-between-liquid-hg-and-vapor-hg-t-c-p-tor-pa556
(Solved): Some vapor pressures of liquid Hg are (equilibrium between liquid Hg and vapor Hg): T (C) P (tor ...
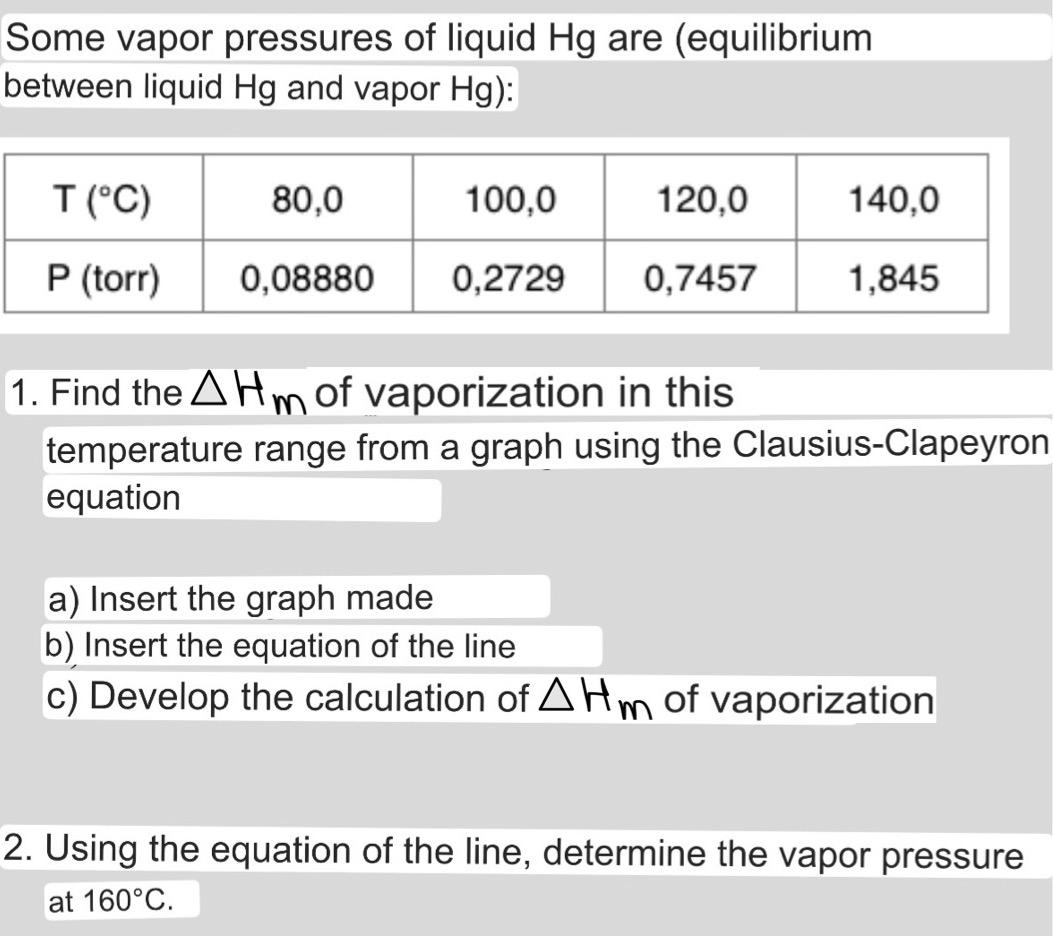
Some vapor pressures of liquid Hg are (equilibrium between liquid Hg and vapor Hg): T (°C) P (torr) 80,0 0,08880 100,0 120,0 0,2729 0,7457 140,0 1,845 1. Find the AHm of vaporization in this temperature range from a graph using the Clausius-Clapeyron equation a) Insert the graph made b) Insert the equation of the line c) Develop the calculation of AHm of vaporization 2. Using the equation of the line, determine the vapor pressure at 160°C.