Home /
Expert Answers /
Chemistry /
solution-of-the-schrodinger-wave-equation-for-the-hydrogen-atom-results-in-a-set-of-functions-orbi-pa394
(Solved): Solution of the Schrodinger wave equation for the hydrogen atom results in a set of functions (orbi ...

Solution of the Schrodinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is charactenized by three quantum numbers: , and . If the value of The quantum number can have values from The total number of orbitals possible at the energy level is If the value of The quantum number / can have values from The total number of orbitals possible at the sublevel is
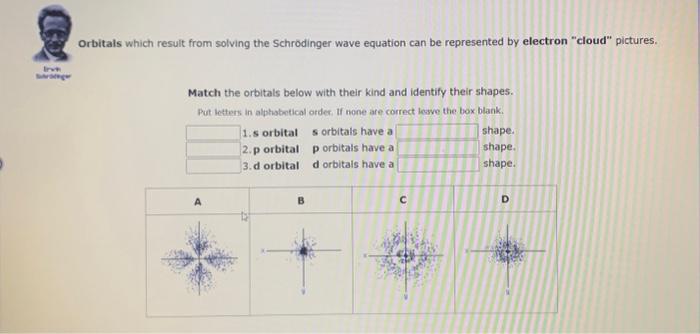
Orbitals which result from solving the Schrodinger wave equation can be represented by electron "cloud" pictures. Match the orbitals below with their kind and identify their shapes. Pot letters in alphabentical order. If none are correct leswe the box blank.
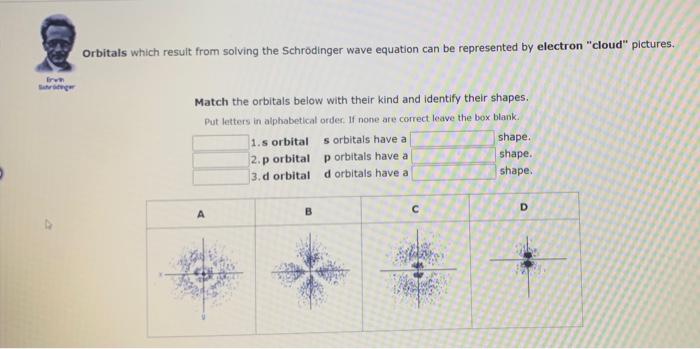
Orbitais which result from solving the Schrodinger wave equation can be represented by electron "cloud" pictures. Match the orbitals below with their kind and identify their shapes. Put letters in alphabetical order. If none are correct leave the box blank. 1.s orbital orbitals have a shape. 2.p orbital orbitals have a shape. 3. d orbital d orbitals have a shape.
Expert Answer
The possible values of l are0 to (n-