Home /
Expert Answers /
Chemistry /
solubility-of-mathrm-agcl-in-ammonia-given-a-g-c-l-s-longleftrightarrow-a-g-a-q-pa232
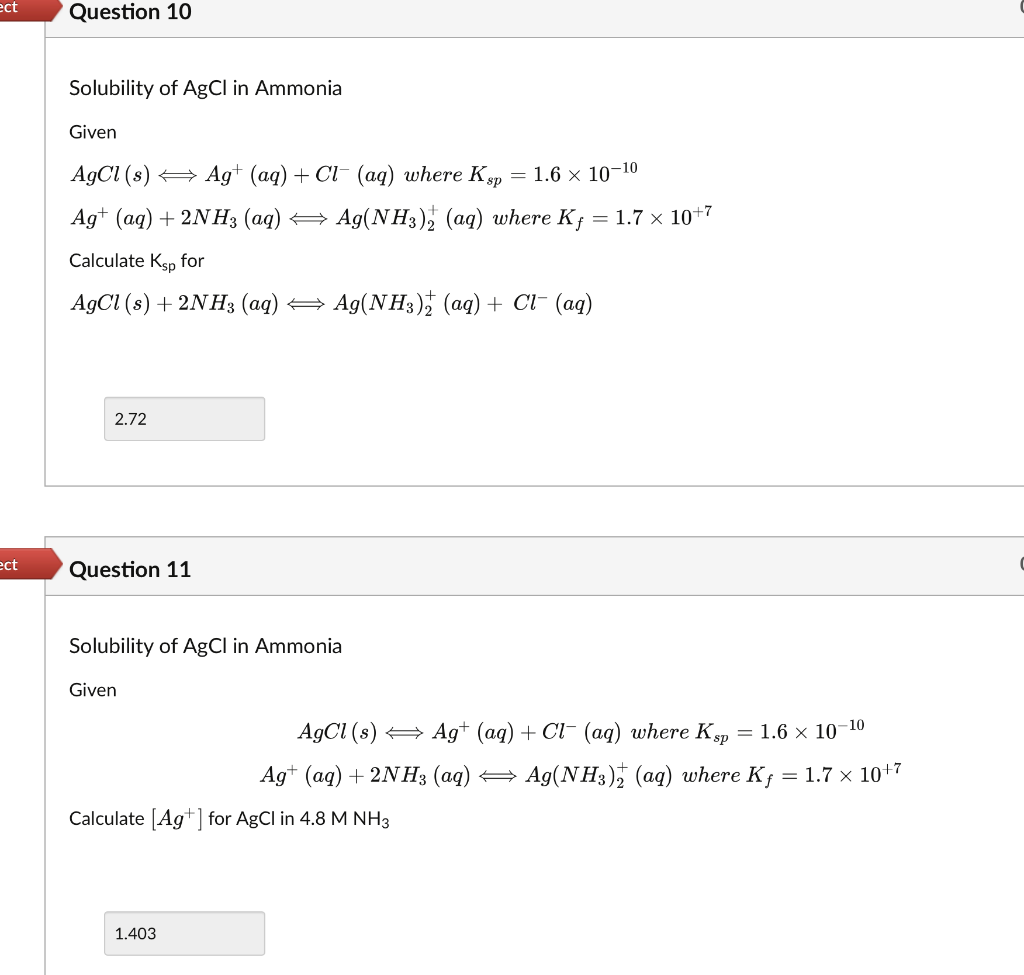
(Solved): Solubility of \( \mathrm{AgCl} \) in Ammonia Given \( A g C l(s) \Longleftrightarrow A g^{+}(a q)+ ...
Solubility of \( \mathrm{AgCl} \) in Ammonia Given \( A g C l(s) \Longleftrightarrow A g^{+}(a q)+C l^{-}(a q) \) where \( K_{s p}=1.6 \times 10^{-10} \) \( \mathrm{Ag}^{+}(a q)+2 \mathrm{NH}_{3}(a q) \Longleftrightarrow A g\left(\mathrm{NH}_{3}\right)_{2}^{+}(a q) \) where \( K_{f}=1.7 \times 10^{+7} \) Calculate \( \mathrm{K}_{\mathrm{sp}} \) for \[ A g C l(s)+2 N_{3}(a q) \Longleftrightarrow A g\left(N H_{3}\right)_{2}^{+}(a q)+C l^{-}(a q) \] Question 11 Solubility of \( \mathrm{AgCl} \) in Ammonia Given \[ \begin{array}{c} A g C l(s) \Longleftrightarrow A g^{+}(a q)+C l^{-}(a q) \text { where } K_{s p}=1.6 \times 10^{-10} \\ A g^{+}(a q)+2 N H_{3}(a q) \Longleftrightarrow A g\left(N H_{3}\right)_{2}^{+}(a q) \text { where } K_{f}=1.7 \times 10^{+7} \end{array} \] Calculate \( \left[\mathrm{Ag}^{+}\right] \)for \( \mathrm{AgCl} \) in \( 4.8 \mathrm{M} \mathrm{NH}_{3} \)