Home /
Expert Answers /
Chemistry /
solid-aluminum-hydrocide-reacts-with-a-solution-of-hydrobromic-acid-write-a-balanced-molecular-equ-pa793
(Solved): Solid aluminum hydrocide reacts with a solution of hydrobromic acid. Write a balanced molecular equ ...
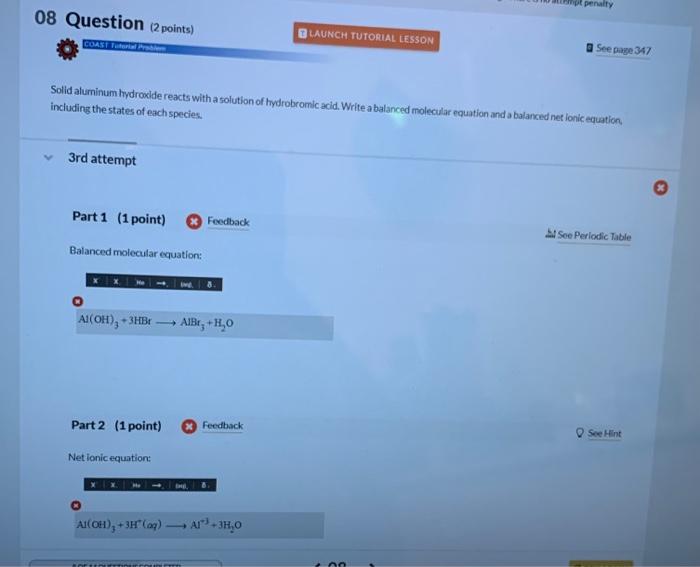
Solid aluminum hydrocide reacts with a solution of hydrobromic acid. Write a balanced molecular equation and a balanced net ionic equation. including the states of each species. attempt Part 1 (1 point) Si Soe Periodic Table Balanced molecular equation: (i) Part 2 (1point) (x) Feedback Net ionic equation: ( )