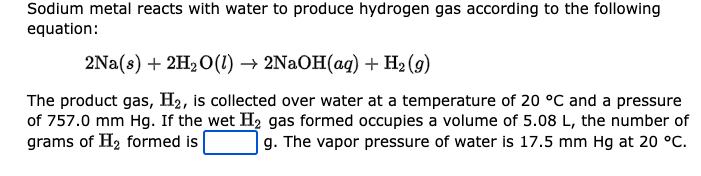Home /
Expert Answers /
Chemistry /
sodium-metal-reacts-with-water-to-produce-hydrogen-gas-according-to-the-following-equation-2-pa869
(Solved): Sodium metal reacts with water to produce hydrogen gas according to the following equation: \[ 2 \ ...
Sodium metal reacts with water to produce hydrogen gas according to the following equation: \[ 2 \mathrm{Na}(s)+2 \mathrm{H}_{2} \mathrm{O}(l) \rightarrow 2 \mathrm{NaOH}(a q)+\mathrm{H}_{2}(g) \] The product gas, \( \mathrm{H}_{2} \), is collected over water at a temperature of \( 20^{\circ} \mathrm{C} \) and a pressure of \( 757.0 \mathrm{~mm} \mathrm{Hg} \). If the wet \( \mathrm{H}_{2} \) gas formed occupies a volume of \( 5.08 \mathrm{~L} \), the number of grams of \( \mathrm{H}_{2} \) formed is g. The vapor pressure of water is \( 17.5 \mathrm{~mm} \mathrm{Hg} \) at \( 20^{\circ} \mathrm{C} \).