Home /
Expert Answers /
Chemistry /
sodium-hydrooide-reacts-with-carbon-dioxide-as-follows-2-mathrm-naoh-s-mathrm-co-2-g-r-pa316
(Solved): Sodium hydrooide reacts with carbon dioxide as follows: \( 2 \mathrm{NaOH}(s)+\mathrm{CO}_{2}(g) \r ...
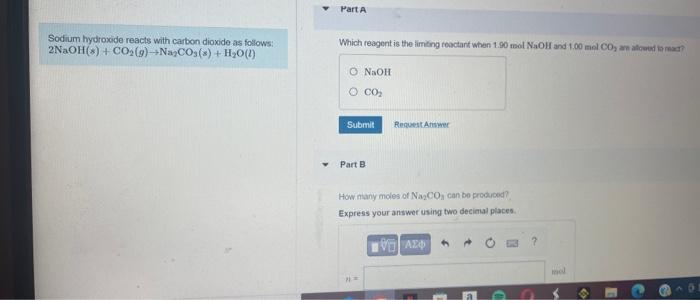
Sodium hydrooide reacts with carbon dioxide as follows: \( 2 \mathrm{NaOH}(s)+\mathrm{CO}_{2}(g) \rightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}(s)+\mathrm{H}_{2} \mathrm{O}(l) \) Which reagent is the limeng roactant whien \( 1.90 \mathrm{~mol} \mathrm{NaOH} \) and \( 1.00 \mathrm{~mol} \mathrm{CO}_{2} \) an atcued armant Part B How many moles of \( \mathrm{Na}_{2} \mathrm{CO}_{a} \) can bo produced? Express your answer using two decimal places.
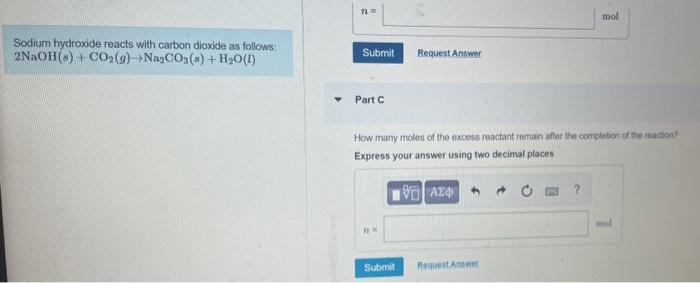
Sodium hydroxide reacts with carbon dioxide as follows: \( 2 \mathrm{NaOH}(s)+\mathrm{CO}_{2}(g) \rightarrow \mathrm{Na}_{2} \mathrm{CO}_{3}(s)+\mathrm{H}_{2} \mathrm{O}(l) \) Part C How many moles of the excess reactant remain after the compleben of the reacicn? Expres5 your answer using two decimal places