Home /
Expert Answers /
Chemistry /
simplified-the-absorption-spectra-of-ions-have-been-used-to-identify-the-presence-of-the-elements-pa410
(Solved): Simplified. The absorption spectra of ions have been used to identify the presence of the elements ...
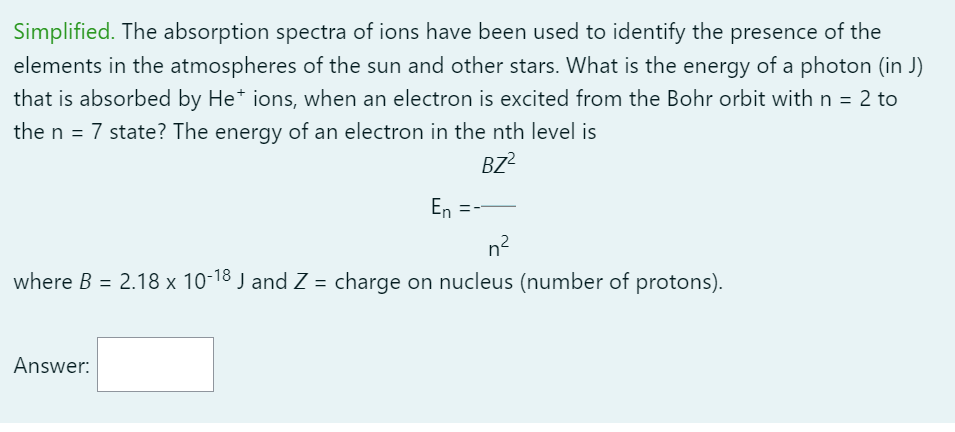
Simplified. The absorption spectra of ions have been used to identify the presence of the elements in the atmospheres of the sun and other stars. What is the energy of a photon (in J) that is absorbed by \( \mathrm{He}^{+} \)ions, when an electron is excited from the Bohr orbit with \( \mathrm{n}=2 \) to the \( \mathrm{n}=7 \) state? The energy of an electron in the \( \mathrm{nth} \) level is \[ \mathrm{E}_{\mathrm{n}}=-\frac{B Z^{2}}{\mathrm{n}^{2}} \] where \( B=2.18 \times 10^{-18} \mathrm{~J} \) and \( Z= \) charge on nucleus (number of protons).