Home /
Expert Answers /
Chemistry /
select-the-correct-lewis-structure-for-mathrm-co-2-i-mathrm-c-mathrm-o-mathrm-pa635
(Solved): Select the correct Lewis structure for \( \mathrm{CO}_{2} \) (i) \( \mathrm{C}: \mathrm{O}: \mathrm ...
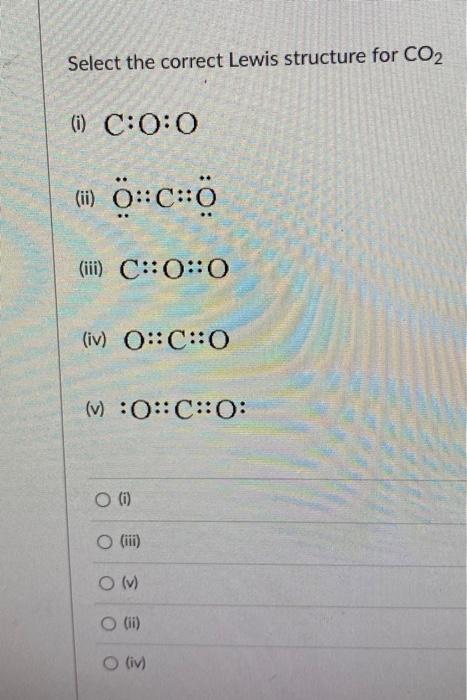

Select the correct Lewis structure for \( \mathrm{CO}_{2} \) (i) \( \mathrm{C}: \mathrm{O}: \mathrm{O} \) (ii) \( \ddot{\mathrm{O}}:: \mathrm{C}:: \ddot{\mathrm{o}} \) (iii) \( \mathrm{C}: \mathrm{O}:: \mathrm{O} \) (iv) \( O:: \mathrm{C}:: \mathrm{O} \) (v) \( : \mathrm{O}: \mathrm{C}:: \mathrm{O}: \) (i) (iii) (v) (ii) (iv)
Order the following elements in order of increasing electronegativity: Ga, S, and Si (i) \( \mathrm{Ga}<\mathrm{S}<\mathrm{Si} \) (ii) \( \mathrm{Si}<\mathrm{S}<\mathrm{Ga} \) (iii) \( \mathrm{S}<\mathrm{Ga}<\mathrm{Si} \) (iv) \( \mathrm{Ga}<\mathrm{Si}<\mathrm{S} \) (v) \( \mathrm{S}<\mathrm{Si}<\mathrm{Ga} \) (i) (v) (iv) (ii) (iii)
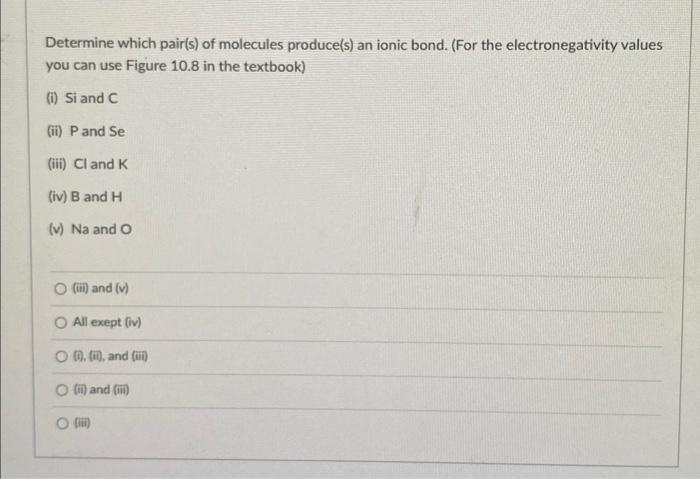
Determine which pair(s) of molecules produce(s) an ionic bond. (For the electronegativity values you can use Figure \( 10.8 \) in the textbook) (i) Si and C (ii) \( \mathrm{P} \) and \( \mathrm{Se} \) (iii) \( \mathrm{Cl} \) and \( \mathrm{K} \) (iv) \( \mathrm{B} \) and \( \mathrm{H} \) (v) \( \mathrm{Na} \) and \( \mathrm{O} \) (iii) and (v) All exept (iv) (i), (ii), and (iii) (ii) and (iii)
Expert Answer
Answer The details of every part is given below. #1 The Lewis structure of carbon dioxide should contain total 16 electrons. There are four balance electron of carbon and 6 balance electron of each oxygen atom. So carbon