Home /
Expert Answers /
Chemistry /
select-correct-lewis-structure-for-mathrm-k-2-mathrm-o-1-mathrm-k-2-0-pa153
(Solved): Select correct Lewis structure for \( \mathrm{K}_{2} \mathrm{O} \). (1) \( [\mathrm{K}]^{2+}[: 0:]^ ...
![Select correct Lewis structure for \( \mathrm{K}_{2} \mathrm{O} \).
(1) \( [\mathrm{K}]^{2+}[: 0:]^{t}[\mathrm{~K}]^{2+} \)
(](https://media.cheggcdn.com/study/160/160a61a0-def8-445b-9e70-ac30dc104ff0/image)
Select correct Lewis structure for \( \mathrm{K}_{2} \mathrm{O} \). (1) \( [\mathrm{K}]^{2+}[: 0:]^{t}[\mathrm{~K}]^{2+} \) (ii) \( [\mathrm{K}]^{++}[: \ddot{\mathrm{O}}:]^{2+}[\mathrm{K}]^{1+} \) (iii) \( [\mathrm{K} \cdot]^{-1+}[: \dot{\mathrm{O}}:]^{-1}[\cdot \mathrm{K}]^{1+} \) (iv) \( [\mathrm{K}:]^{+}[: \mathrm{O}:]^{2+}[: \mathrm{K}]^{+} \) (v) \( [\mathrm{K}:]^{+}[\ddot{O} \cdot:]^{2+}[: \mathrm{K}]^{+} \) (i) (iv) (v)
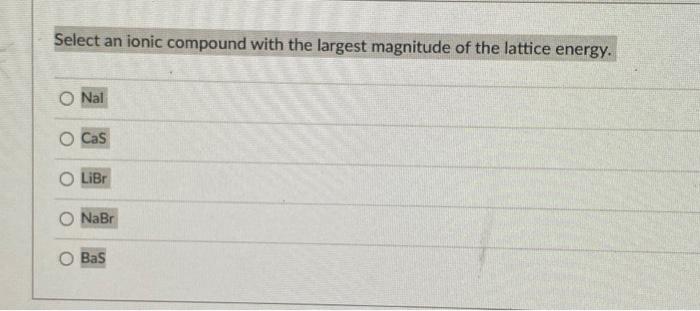
Select an ionic compound with the largest magnitude of the lattice energy. Nal CaS LiBr \( \mathrm{NaBr} \) BaS
Expert Answer
K2O is ionic compound in which - Cation - K+ (As p