Home /
Expert Answers /
Chemistry /
select-all-that-apply-the-reaction-and-rate-law-for-the-gas-phase-decomposition-of-dinitrogen-pent-pa624
(Solved): Select all that apply. The reaction and rate law for the gas-phase decomposition of dinitrogen pent ...
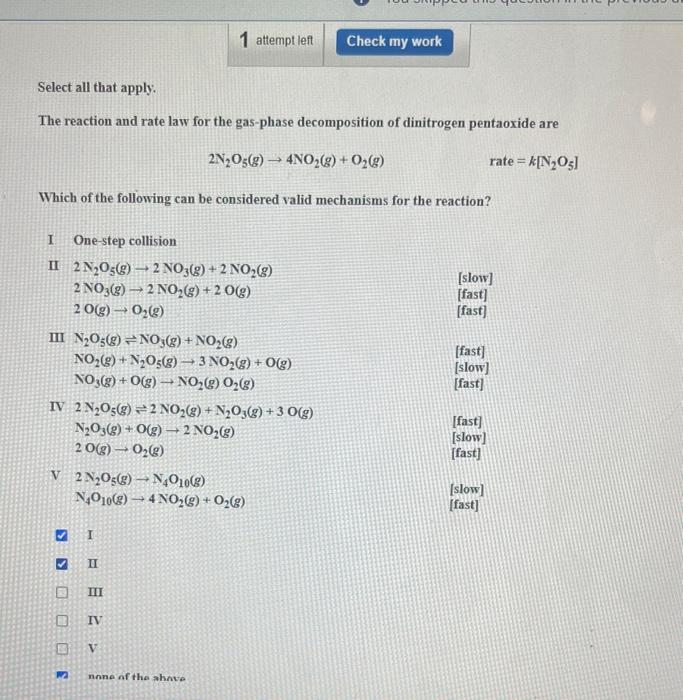
Select all that apply. The reaction and rate law for the gas-phase decomposition of dinitrogen pentaoxide are 2N?O(g)? 4NO?(g) + O?(g) rate=k[N?05] Which of the following can be considered valid mechanisms for the reaction? I II 2 N?O(g) 2 NO3(g) 20(g) ? One-step collision III N?O(g) NO3(g) + NO?(g) S B 12 V 2N?O(g) N4O10(g) IV 2 N?O(g) 2 NO?(g) + N?O3(g) + 3 0(g) N?O3(g) + O(g) ? 2 NO?(g) 20(g) ? O?(g) NO?(g) + N?O5(g) ? 3 NO?(g) + O(g) - O?(g) NO3(g) + O(g) ? NO?(g) O?(g) - I 2 NO3(g) +2 NO?(g) 2 NO?(g) + 2 O(g) II III 1 attempt left IV V N4010(g) 4NO?(g) + O?(g) none of the above Check my work [slow] [fast] [fast] [fast] [slow] [fast] [fast] [slow] [fast] [slow] [fast]