Home /
Expert Answers /
Chemistry /
schematic-diagrams-of-three-open-tube-mercury-manometers-are-shown-below-not-to-scale-suppose-t-pa822
(Solved): Schematic diagrams of three open-tube mercury manometers are shown below (not to scale). Suppose t ...
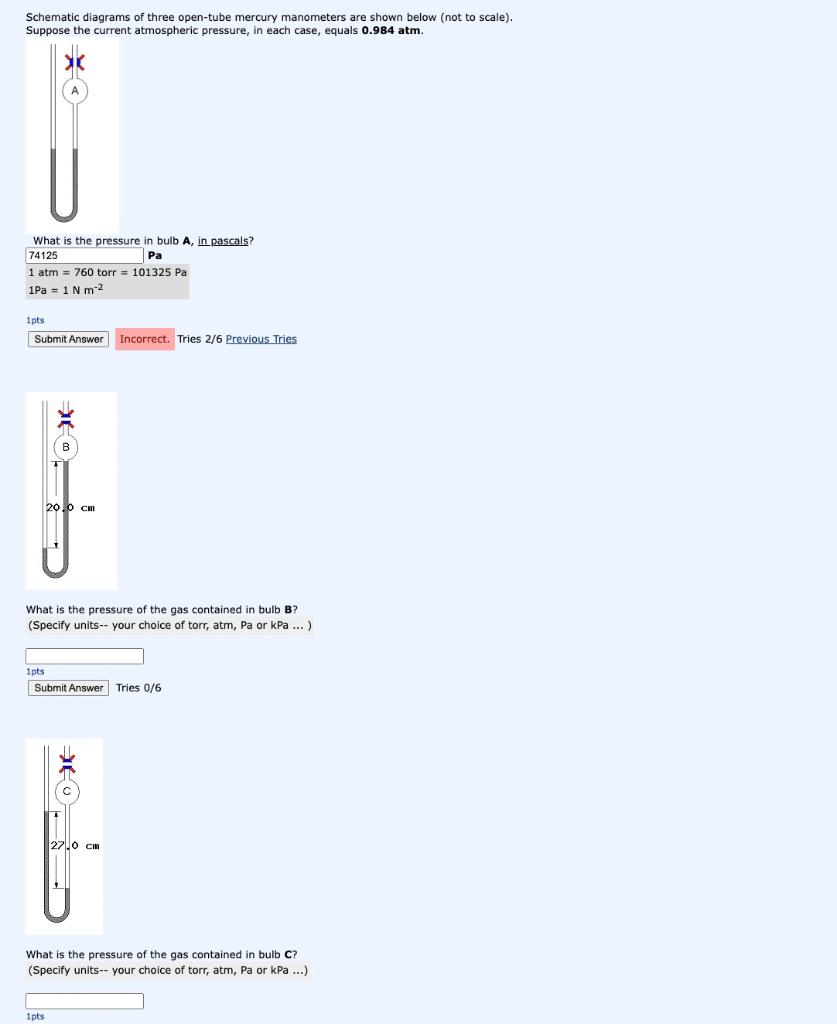
Schematic diagrams of three open-tube mercury manometers are shown below (not to scale). Suppose the current atmospheric pressure, in each case, equals 0.984 atm. What is the pressure in bulb A, in pascals? 74125 Pa 1 atm = 760 torr = 101325 Pa 1Pa = 1 N m-2 1pts Submit Answer Incorrect. Tries 2/6 Previous Tries 20.0 cm What is the pressure of the gas contained in bulb B? (Specify units-- your choice of torr, atm, Pa or kPa... ) 1pts Submit Answer Tries 0/6 27.0 cm What is the pressure of the gas contained in bulb C? (Specify units-- your choice of torr, atm, Pa or kPa...) 1pts #F
Expert Answer
BULB A : In both arms of the manometers the mercury levels are at same height. This is possible when the pressure inside the bulb (say Pgas) is equal to atmospheric pressure (say Patm). So in this case, Pgas = Patm = 0.984 atm = (0.984 x 101325) Pa =