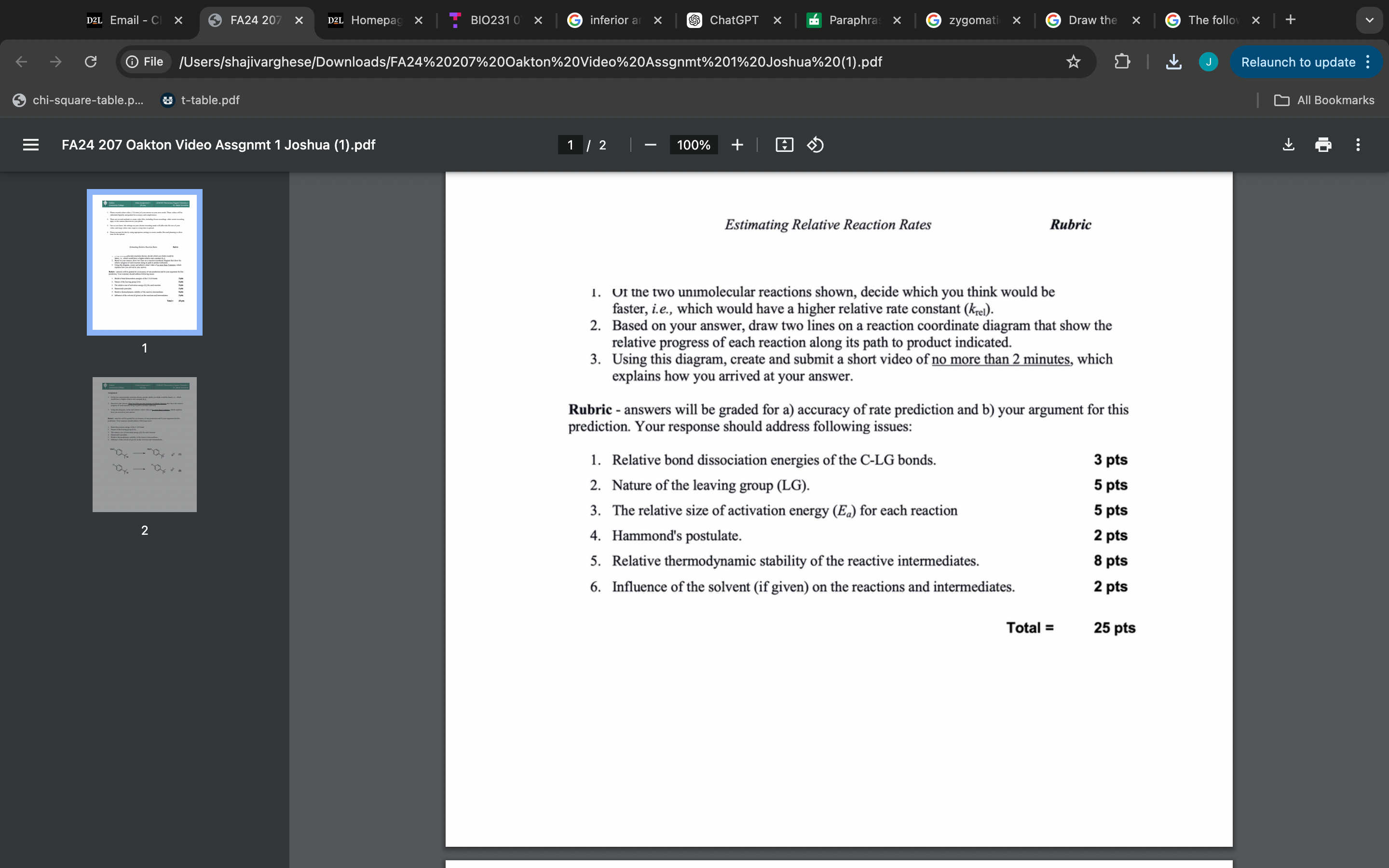
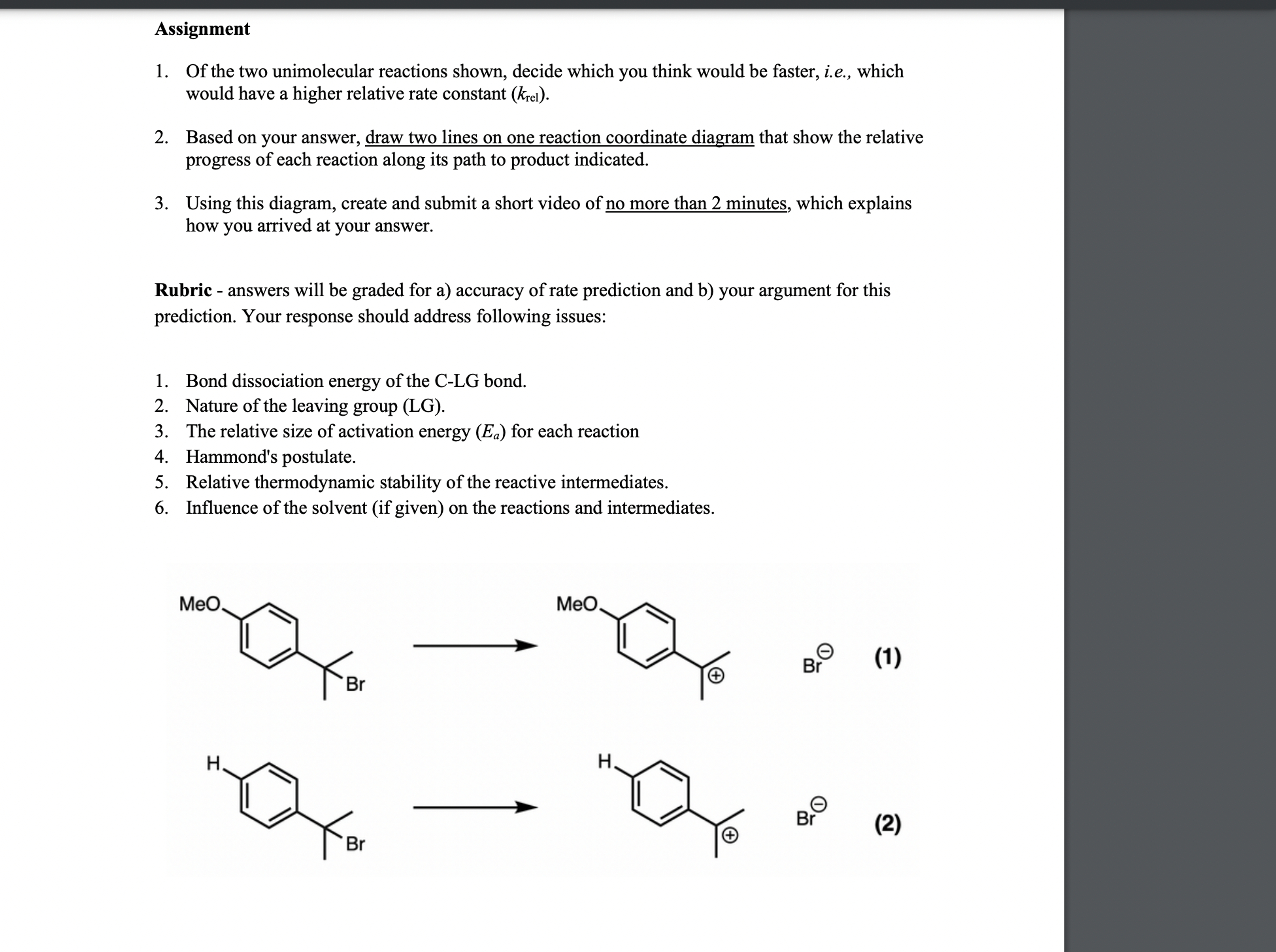
(Solved): Rubric 1. Ut the two unmolecular reactions shown, decide which you think would be faster, i.e., wh ...
Rubric 1. Ut the two un?molecular reactions shown, decide which you think would be faster, i.e., which would have a higher relative rate constant ( \( \left.k_{\mathrm{rel}}\right) \). 2. Based on your answer, draw two lines on a reaction coordinate diagram that show the relative progress of each reaction along its path to product indicated. 3. Using this diagram, create and submit a short video of no more than 2 minutes, which explains how you arrived at your answer. Rubric - answers will be graded for a) accuracy of rate prediction and b) your argument for this prediction. Your response should address following issues: 1. Relative bond dissociation energies of the C-LG bonds. 2. Nature of the leaving group (LG). 3. The relative size of activation energy \( \left(E_{a}\right) \) for each reaction 4. Hammond's postulate. 5. Relative thermodynamic stability of the reactive intermediates. 6. Influence of the solvent (if given) on the reactions and intermediates. Assignment 1. Of the two unimolecular reactions shown, decide which you think would be faster, i.e., which would have a higher relative rate constant \( \left(k_{\mathrm{rel}}\right) \). 2. Based on your answer, draw two lines on one reaction coordinate diagram that show the relative progress of each reaction along its path to product indicated. 3. Using this diagram, create and submit a short video of no more than 2 minutes, which explains how you arrived at your answer. Rubric - answers will be graded for a) accuracy of rate prediction and b) your argument for this prediction. Your response should address following issues: 1. Bond dissociation energy of the C-LG bond. 2. Nature of the leaving group (LG). 3. The relative size of activation energy \( \left(E_{a}\right) \) for each reaction 4. Hammond's postulate. 5. Relative thermodynamic stability of the reactive intermediates. 6. Influence of the solvent (if given) on the reactions and intermediates. \[ \longrightarrow \] (2)