Home /
Expert Answers /
Chemistry /
report-sheet-the-molarity-of-hydrochloric-acid-molarity-of-naoh-0-333-moles-liter-trial-unknown-ac-pa310
(Solved): REPORT SHEET: The Molarity of Hydrochloric Acid Molarity of NaOH: 0.333 moles/liter Trial Unknown ac ...
REPORT SHEET: The Molarity of Hydrochloric Acid Molarity of NaOH: 0.333 moles/liter Trial Unknown acid number: N/A 9.75 ml Volume of HCI sample: Final buret reading, NaOH: 3.20 ml Initial buret reading, NaOH: 8.33 ml Volume of NaOH required to reach the endpoint: Experiment 16 You calculate this: Acld-Base Titrations Trial 2 9.80 ml 3.25 ml 8.38 ml You calculate this: 2
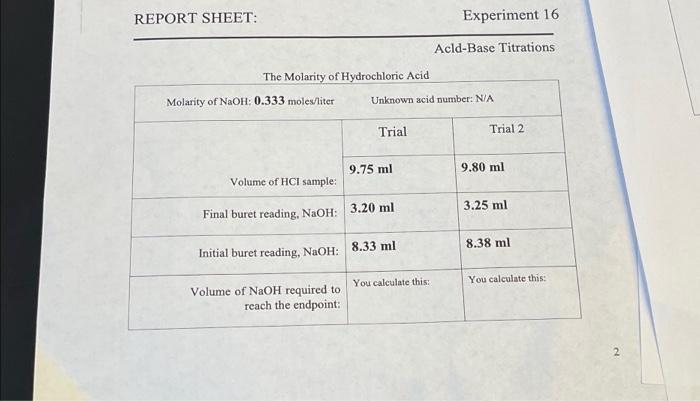
REPORT SHEET: Experiment 16 Acld-Base Titrations The Molarity of Hydrochloric Acid \begin{tabular}{|c|c|c|} \hline Molarity of moles/iter & Unknown acid & nber: N/A \\ \hline \multirow[b]{2}{*}{ Volume of sample: } & Trial & Trial 2 \\ \hline & & \\ \hline Final buret reading, : & & \\ \hline Initial buret reading, : & & \\ \hline \begin{tabular}{l} Volume of required to \\ reach the endpoint: \end{tabular} & You calculate this: & You calculate this: \\ \hline \end{tabular} 2