Home /
Expert Answers /
Chemistry /
report-no-4-formulas-and-nomenclaturepart-1-part-3-give-the-cation-anion-and-formula-for-each-o-pa686
(Solved): REPORT No. 4: Formulas and nomenclaturePart 1:part 3: Give the cation, anion, and formula for each o ...
REPORT No. 4: Formulas and nomenclature








Part 1:

part 3:


Give the cation, anion, and formula for each of the following ionic compounds. The first is done as an example. Give the name for each of the following ionic compounds. \( \mathrm{NH}_{4} \mathrm{ClO}_{3} \) \( \mathrm{Mg}\left(\mathrm{ClO}_{4}\right)_{2} \) \( \mathrm{Pb}\left(\mathrm{CH}_{3} \mathrm{CO}_{2}\right)_{4} \) \( \mathrm{AgSCN} \) \( \mathrm{K}_{2} \mathrm{SO}_{4} \) \( \mathrm{CuHSO}_{4} \) Give an example (f an ionic compound (formula and name) for each of the following. A \( +1 \) transition metal and a -2 polyatomic ion. A \( +2 \) main group metal and \( -3 \) polyatomic ion.
Write the formula for each of the following covalent compounds. xenon hexafluoride selenium dichloride silicon tetrachloride arsenic pentafluoride dinitrogen tetroxide tetrasulfur tetranitride disulfur heptoxide. carbonic acid Give the name for each of the following covalent compounds. All acids are aqueous. \( \mathrm{H}_{2} \mathrm{SO}_{4} \) \( \mathrm{N}_{2} \mathrm{O}_{5} \) \( \mathrm{SO}_{3} \) \( \mathrm{B}_{2} \mathrm{~F}_{4} \) \( \mathrm{SiO} \) \( \mathrm{CS}_{2} \) \( \mathrm{P}_{4} \mathrm{O}_{10} \) \( \mathrm{SiCl}_{4} \)
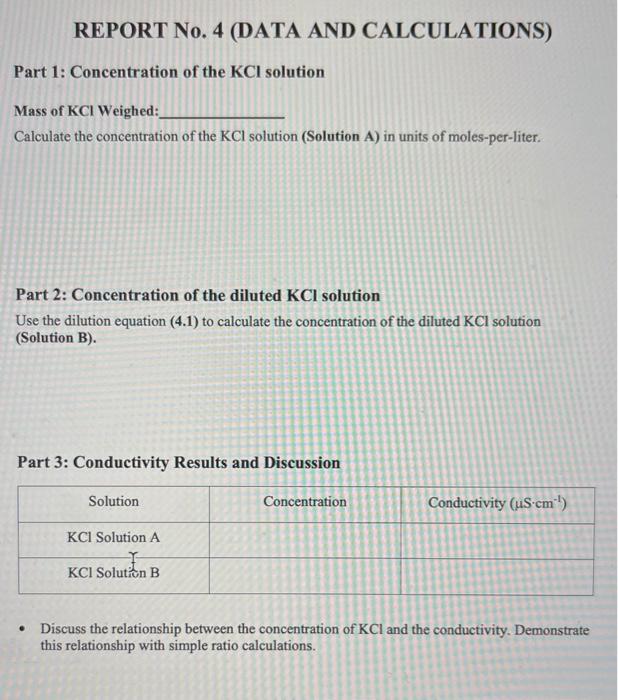
Part 1: Concentration of the \( \mathrm{KCl} \) solution Mass of \( \mathrm{KCl} \) Weighed: Calculate the concentration of the \( \mathrm{KCl} \) solution (Solution A) in units of moles-per-liter. Part 2: Concentration of the diluted \( \mathrm{KCl} \) solution Use the dilution equation (4.1) to calculate the concentration of the diluted \( \mathrm{KCl} \) solution (Solution B). Part 3: Conductivity Results and Discussion - Discuss the relationship between the concentration of \( \mathrm{KCl} \) and the conductivity. Demonstrate this relationship with simple ratio calculations.
This data set contains the mass readings for the solid sample of \( \mathrm{KCl} \) needed to make Solution \( \mathrm{A} \). Refer to the Laboratory Manual for reference. Mass of \( \mathrm{KCl} \) sample used to prepare Solution A.
This data set contains the conductivity readings for the concentrated and dilute solutions of \( \mathrm{KCl} \). Refer to the Laboratory Manual for reference. Conductivity reading of the concentrated \( \mathrm{KCl} \) solution (Solution A).
Conductivity reading of the dilute \( \mathrm{KCl} \) solution (Solution B).
Expert Answer
Formula of the following compounds are as follows: Name of the following ionic