Home /
Expert Answers /
Chemistry /
record-the-correct-number-of-significant-figures-and-units-wilh-your-measurements-1-over-the-cou-pa980
(Solved): Record the correct number of significant figures and units wilh your measurements. 1. Over the cou ...
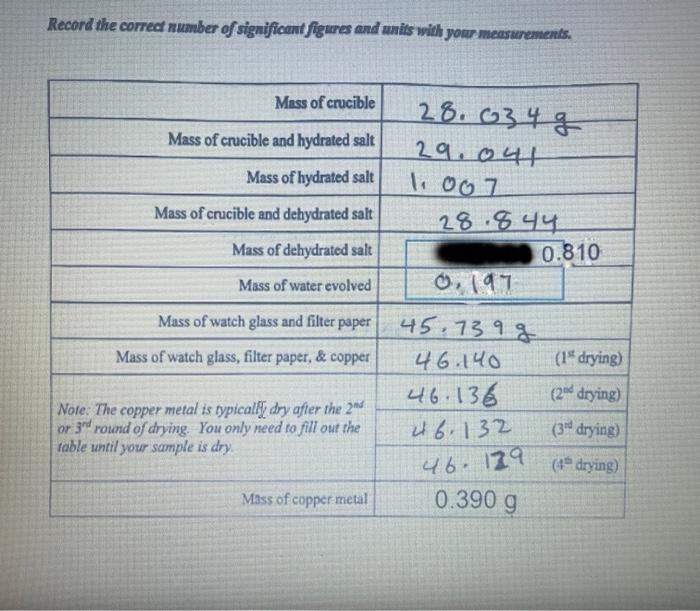


Record the correct number of significant figures and units wilh your measurements.
1. Over the course of the experiment the copper and waters of hydration present in the copper chloride hydrate were isolated and the mass of each can be determined from your data. Using these mass measurements, determine the expected mass of chloride ion remaining in the solution. 2. Determine the number of moles of each species listed below present in your copper chloride hydrate sample. a. Copper ion: b. Chloride ion: c. Waters of Hydrate: 3. Using your data, determine the chemical formula of your copper chloride salt. No creait for this question will be awarded without work.
4. Throughout the experiment you were instructed to quantitatively transfer your sample using aliquots of DI water. Why? 5. How would your measurement of the mass, and number of moles, of the copper metal change if the copper metal wasn't completely dry (i.e. would it increase, decrease, or stay the same)? 6. How would the determined mass, and number of moles, of the chloride ion change if the copper metal wasn't completely dry (i.e. would it increase decrease, or stay the same)? 7. Copper typically forms salts as copper(I) or copper(II) cations. Copper chloride salts are typically either anhydrous (no water) or dihydrates. Given this information, is the formula you calculated in question 3 reasonable?