Home /
Expert Answers /
Chemistry /
reagents-isoleucine-the-transformation-above-can-be-performed-with-some-reagent-or-combination-of-t-pa493
(Solved): reagents Isoleucine The transformation above can be performed with some reagent or combination of t ...
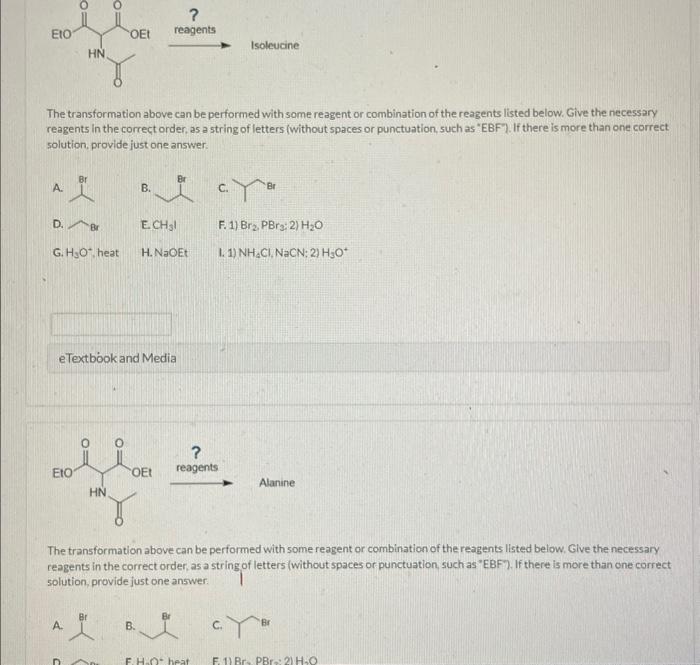

reagents Isoleucine The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order. as a string of letters (without spaces or punctuation, such as "EBF". If there is more than one correct. solution, provide just one answer. A. c. D. Br E. \( \mathrm{CH}_{3} \mathrm{I} \) F. 1) \( \mathrm{Br}_{2}, \mathrm{PBr}_{3} ; \) 2) \( \mathrm{H}_{2} \mathrm{O} \) G. \( \mathrm{H}_{3} \mathrm{O}^{+} \), heat H. NaOEt 1. 1) \( \mathrm{NH}_{4} \mathrm{Cl}_{,} \mathrm{NaCN} \) : 2) \( \mathrm{H}_{3} \mathrm{O}^{+} \) eTextbook and Media reagents The transformation above can be performed with some reagent or combination of the reagents listed below. Glve the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF7). If there is more than one correct solution, provide just one answer. A. B. c.
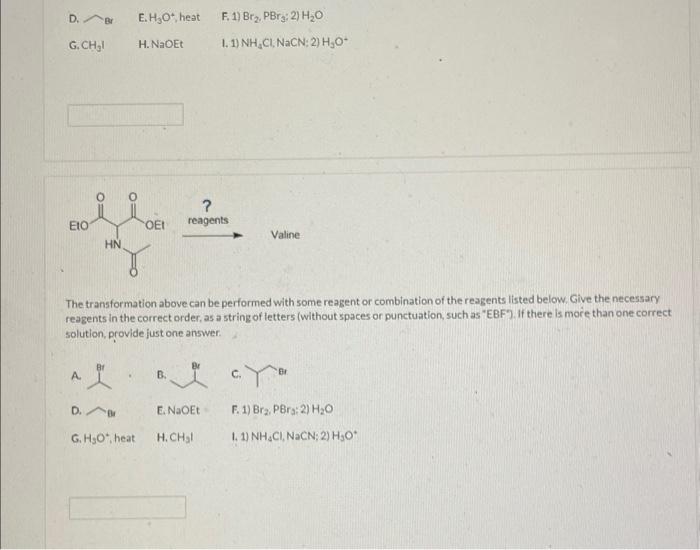
reagents The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide fust one answer.
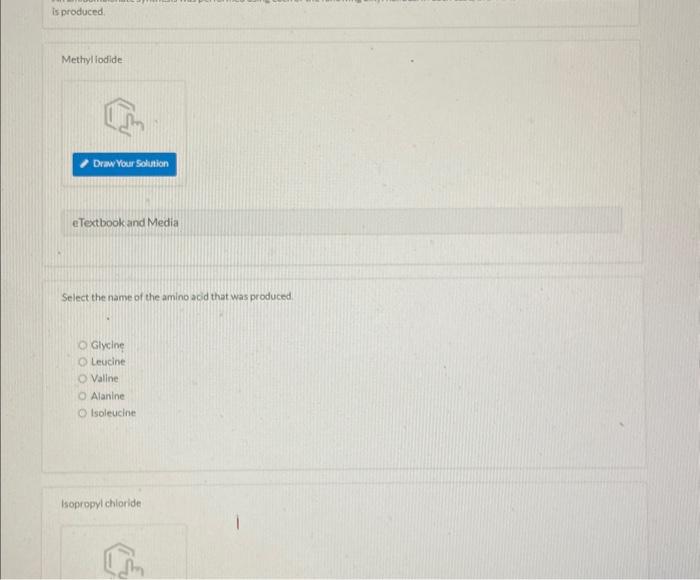
Methyl lodide eTextbook and Media Select the name of the amino acid that was produced: Gilycine Lecicine Valine Alanine Isoleucliet
Both leucine and isoleucine can be prepared via the amidomalonate synthesis, although one of these amino acids can be produced in higher yields. Identify the higher yielding process and explain your choice. Isoleucine is produced in higher yields because the required alkyl halide is primary, resulting in a more efficient \( S_{N} 2 \) process. Leucine is produced in higher yields because the required alkyl halide is primary, resulting in a more efficient \( S_{N} 2 \) process. Leucine is produced in higher yields because the required alkyl halide is secondary, resulting in a more efficient \( S_{N}{ }^{2} \) process. Isoleucine is produced in higher yields because the required alkyl halide is secondary, resulting in a more efficient \( S_{N}{ }^{2} \) process.
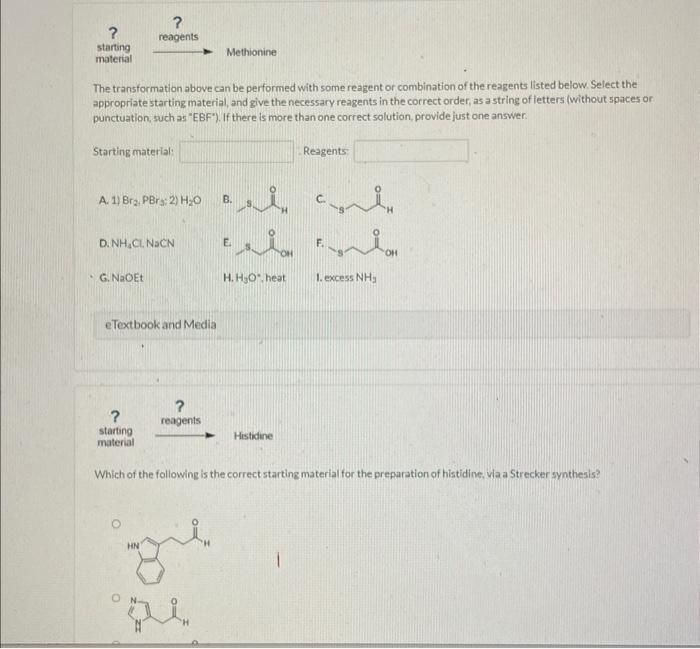
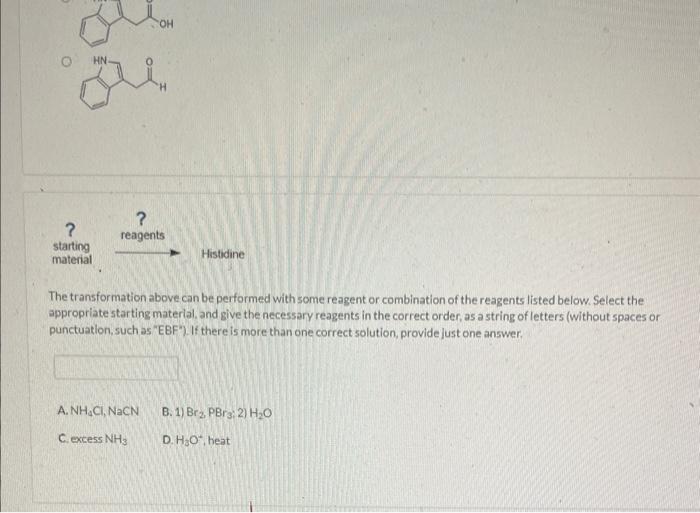
The transformation above can be performed with some reagent or combination of the reagents listed below. Select the appropriate starting material, and give the necessary reagents in the correct order, as a string of letters (without spaces or. punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. Starting material: Reggents: A. 1) \( \mathrm{Br}_{2}, \mathrm{PBr} ; \); \( 2 \mathrm{H}_{2} \mathrm{O} \) D. \( \mathrm{NH}_{4} \mathrm{Cl}, \mathrm{NaCN} \) G. NaOEt H. \( \mathrm{H}_{3} \mathrm{O}^{\circ} \), heat I. excess \( \mathrm{NH}_{3} \) Which of the following is the correct starting material for the preparation of histidine. Wla a Strecker synthesis?
The transformation above can be performed with some reagent or combination of the reagents listed below. Select the appropriate starting material, and give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. \( \mathrm{NH}_{4} \mathrm{Cl}, \mathrm{NaCN} \) B. 1) \( \mathrm{Br}_{2}, \mathrm{PBr}_{3} ; \) 2) \( \mathrm{H}_{2} \mathrm{O} \) C. excess \( \mathrm{NH}_{3} \) D. \( \mathrm{H}_{3} \mathrm{O}^{4} \), heat
Expert Answer
Q-01 ; step-01 ; NaOEt , strong base abstract proton from active methylene group gives rise to carbanion intermediate. step-02 ; Sn2 type reaction with carbanion intermediate with alkyl bromide, 2-bromobutane yield to alkylated product . Step-03 ; hy