Home /
Expert Answers /
Chemistry /
reaction-progress-use-the-reaction-energy-diagram-above-to-answer-the-following-questions-cal-pa831
(Solved): Reaction progress Use the reaction energy diagram above to answer the following questions. Cal ...
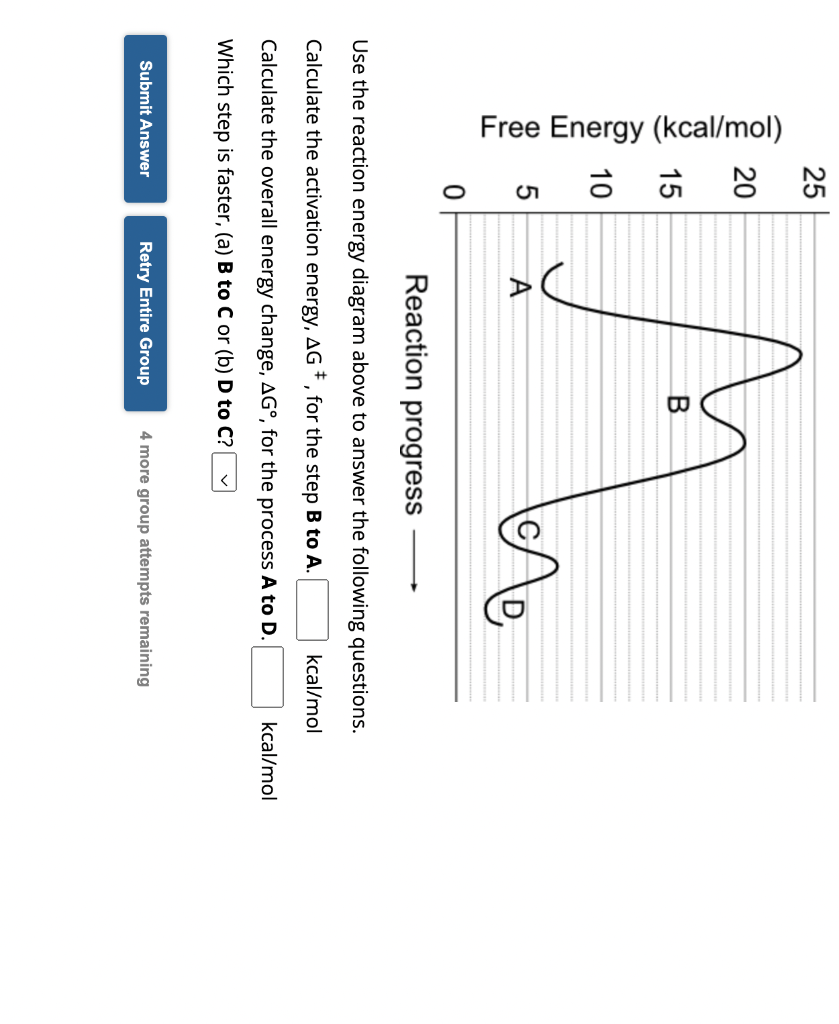
Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, , for the step to Calculate the overall energy change, , for the process to . Which step is faster, (a) B to C or (b) D to C?
Expert Answer
To calculate activation energy and change in Gibbs free energy from the energy profile diagram, you need to understand the basic concepts behind these two quantities.Activation energy (Ea) is the energy required to initiate a chemical reaction. It is typically measured as the difference in energy between the reactants and the transition state of the reaction.On the other hand, the change in Gibbs free energy (?G) is a measure of the spontaneity of a chemical reaction. It is calculated as the difference in the Gibbs free energy of the products and the reactants: