Home /
Expert Answers /
Chemistry /
reaction-mechanism-for-the-following-reaction-preparation-of-n-acetyl-l-prolyl-l-phenylalan-pa737
(Solved): Reaction mechanism for the following reaction Preparation of \( N \)-acetyl-L-prolyl-L-phenylalan ...
Reaction mechanism for the following reaction
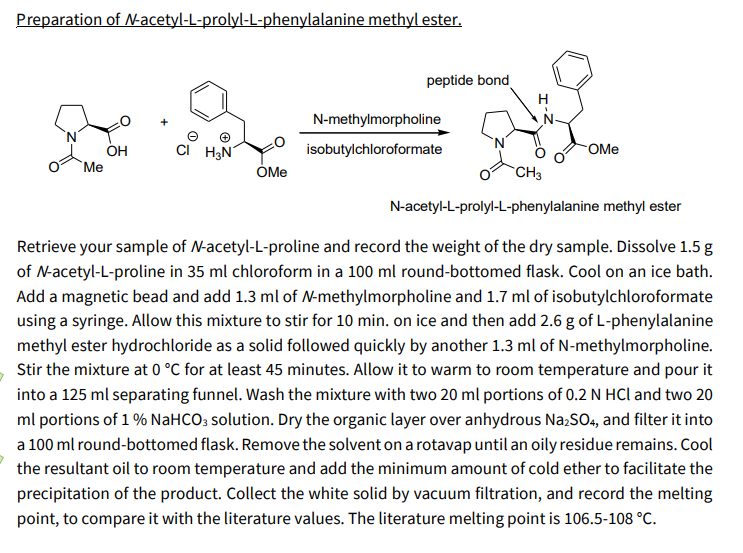
Preparation of \( N \)-acetyl-L-prolyl-L-phenylalanine methyl ester. peptide bond \( \underset{\text { isobutylchloroformate }}{\stackrel{\mathrm{N} \text {-methylmorpholine }}{\longrightarrow}} \) \( \mathrm{N} \)-acetyl-L-prolyl-L-phenylalanine methyl ester Retrieve your sample of \( N \)-acetyl-L-proline and record the weight of the dry sample. Dissolve \( 1.5 \mathrm{~g} \) of \( \mathrm{N} \)-acetyl-L-proline in \( 35 \mathrm{ml} \) chloroform in a \( 100 \mathrm{ml} \) round-bottomed flask. Cool on an ice bath. Add a magnetic bead and add \( 1.3 \mathrm{ml} \) of \( \mathrm{N} \)-methylmorpholine and \( 1.7 \mathrm{ml} \) of isobutylchloroformate using a syringe. Allow this mixture to stir for \( 10 \mathrm{~min} \). on ice and then add \( 2.6 \mathrm{~g} \) of L-phenylalanine methyl ester hydrochloride as a solid followed quickly by another \( 1.3 \mathrm{ml} \) of \( \mathrm{N} \)-methylmorpholine. Stir the mixture at \( 0{ }^{\circ} \mathrm{C} \) for at least 45 minutes. Allow it to warm to room temperature and pour it into a \( 125 \mathrm{ml} \) separating funnel. Wash the mixture with two \( 20 \mathrm{ml} \) portions of \( 0.2 \mathrm{~N} \mathrm{HCl} \) and two 20 \( \mathrm{ml} \) portions of \( 1 \% \mathrm{NaHCO}_{3} \) solution. Dry the organic layer over anhydrous \( \mathrm{Na}_{2} \mathrm{SO}_{4} \), and filter it into a \( 100 \mathrm{ml} \) round-bottomed flask. Remove the solvent on a rotavap until an oily residue remains. Cool the resultant oil to room temperature and add the minimum amount of cold ether to facilitate the precipitation of the product. Collect the white solid by vacuum filtration, and record the melting point, to compare it with the literature values. The literature melting point is \( 106.5-108^{\circ} \mathrm{C} \).