Home /
Expert Answers /
Chemistry /
questions-1-draw-the-lewis-dot-structure-of-methyl-isocyanate-mic-2-label-all-of-the-polar-cov-pa526
(Solved): Questions 1. Draw the Lewis dot structure of methyl isocyanate (MIC). 2. Label all of the polar cov ...
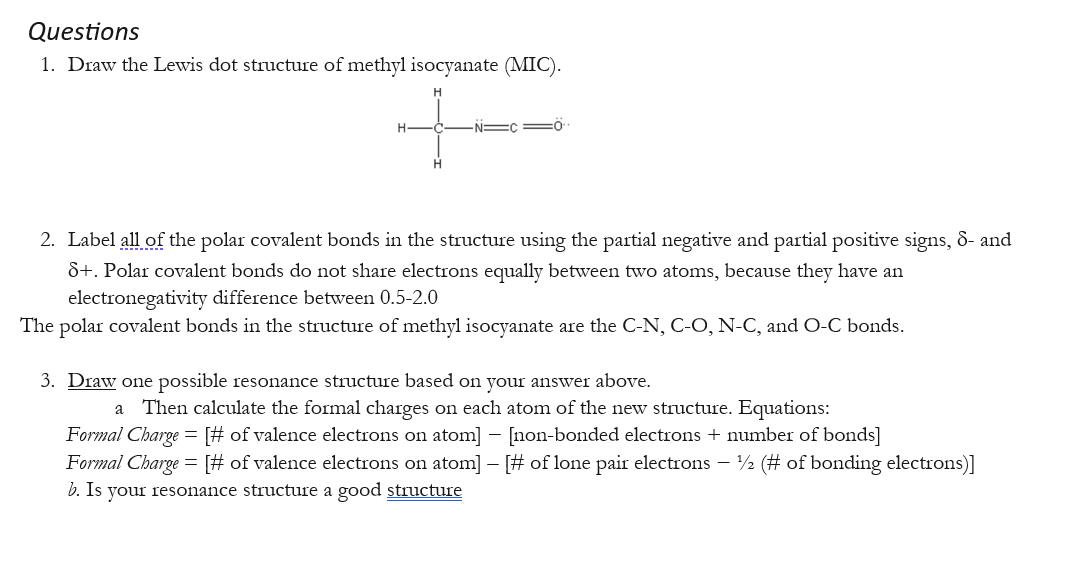
Questions 1. Draw the Lewis dot structure of methyl isocyanate (MIC). 2. Label all of the polar covalent bonds in the structure using the partial negative and partial positive signs, - and . Polar covalent bonds do not share electrons equally between two atoms, because they have an electronegativity difference between The polar covalent bonds in the structure of methyl isocyanate are the , and bonds. 3. Draw one possible resonance structure based on your answer above. a Then calculate the formal charges on each atom of the new structure. Equations: Formal Charge [\# of valence electrons on atom [non-bonded electrons + number of bonds Formal Charge of valence electrons on atom of lone pair electrons (\# of bonding electrons) . Is your resonance structure a good structure