Home /
Expert Answers /
Chemistry /
question-7-aldehydes-have-higher-boiling-points-than-alkanes-of-similar-molar-mass-because-of-o-dip-pa150
(Solved): QUESTION 7 Aldehydes have higher boiling points than alkanes of similar molar mass because of O dip ...
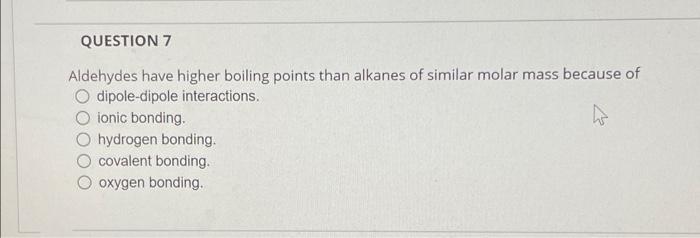

QUESTION 7 Aldehydes have higher boiling points than alkanes of similar molar mass because of O dipole-dipole interactions. ionic bonding. hydrogen bonding. covalent bonding. Ooxygen bonding..
QUESTION 6 Aldehyldes have a higher boiling point than alcohols. O True because aldehydes can undergo hydrogen bonding with themselves, while alcohols cannot. True because alcohols can undergo hydrogen bonding with themselves, while aldehydes cannot. False because alcohols can undergo hydrogen bonding with themselves, wille aldehydes cannot. False because aldehydes can undergo hydrogen bonding with themselves, while alcohols cannot.
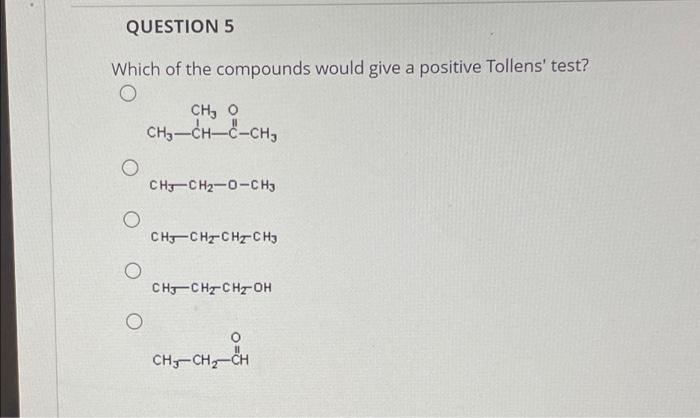
QUESTION 5 Which of the compounds would give a positive Tollens' test? O O O O O CH, O CH?-CH-C-CH? CH3CH?-O-CH3 CH3CH?CH?CH3 CH3CH2CH?-OH CH-CH?-CH
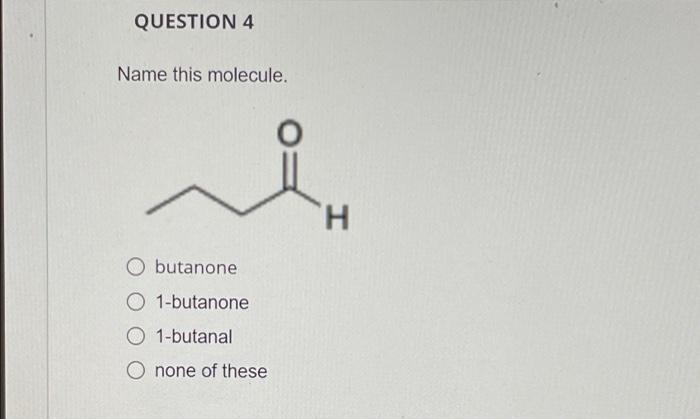
QUESTION 4 Name this molecule. O butanone O 1-butanone O 1-butanal O none of these H
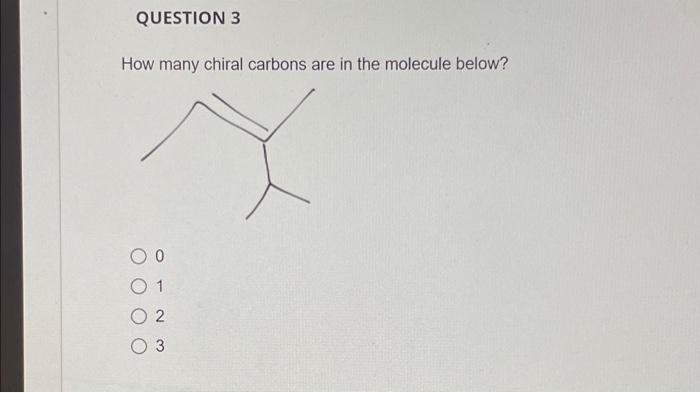
QUESTION 3 How many chiral carbons are in the molecule below? O 0 1 02 3
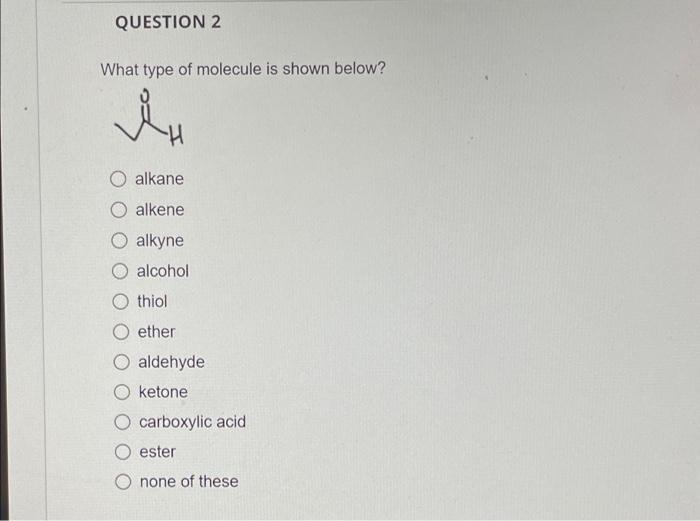
QUESTION 2 What type of molecule is shown below? i H O alkane O alkene O alkyne O alcohol thiol ether aldehyde O ketone carboxylic acid ester none of these